Biodistribution and Pharmacokinectics of Liposomes and Exosomes in a Mouse Model of Sepsis
- PMID: 33809966
- PMCID: PMC8004782
- DOI: 10.3390/pharmaceutics13030427
Biodistribution and Pharmacokinectics of Liposomes and Exosomes in a Mouse Model of Sepsis
Abstract
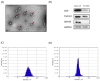
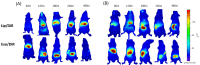
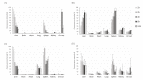
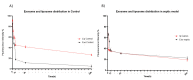
Exosomes have attracted considerable attention as drug delivery vehicles because their biological properties can be utilized for selective delivery of therapeutic cargoes to disease sites. In this context, analysis of the in vivo behaviors of exosomes in a diseased state is required to maximize their therapeutic potential as drug delivery vehicles. In this study, we investigated biodistribution and pharmacokinetics of HEK293T cell-derived exosomes and PEGylated liposomes, their synthetic counterparts, into healthy and sepsis mice. We found that biodistribution and pharmacokinetics of exosomes were significantly affected by pathophysiological conditions of sepsis compared to those of liposomes. In the sepsis mice, a substantial number of exosomes were found in the lung after intravenous injection, and their prolonged blood residence was observed due to the liver dysfunction. However, liposomes did not show such sepsis-specific effects significantly. These results demonstrate that exosome-based therapeutics can be developed to manage sepsis and septic shock by virtue of their sepsis-specific in vivo behaviors.
Keywords: biodistribution; exosome; liposome; near-infrared imaging; pharmacokinetics; sepsis.
Conflict of interest statement
The authors declare no conflict of interest. C.C is an inventor of a patent related to this work filed by ILIAS Biologics Inc. (no. KR 10-1877010 and US 10702581). C.C is the founder and shareholder, and S.H.A is minor shareholders of ILIAS Biologics Inc.
Figures
References
-
- Yim N., Ryu S.W., Choi K., Lee K.R., Lee S., Choi H., Kim J., Shaker M.R., Sun W., Park J.H., et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat. Commun. 2016;7:12277. doi: 10.1038/ncomms12277. - DOI - PMC - PubMed
-
- Yáñez-Mó M., Siljander P.R.M., Andreu Z., Zavec A.B., Borràs F.E., Buzas E.I., Buzas K., Casal E., Cappello F., Carvalho J., et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

