Key Matrix Remodeling Enzymes: Functions and Targeting in Cancer
- PMID: 33809973
- PMCID: PMC8005147
- DOI: 10.3390/cancers13061441
Key Matrix Remodeling Enzymes: Functions and Targeting in Cancer
Abstract
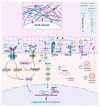
Tissue functionality and integrity demand continuous changes in distribution of major components in the extracellular matrices (ECMs) under normal conditions aiming tissue homeostasis. Major matrix degrading proteolytic enzymes are matrix metalloproteinases (MMPs), plasminogen activators, atypical proteases such as intracellular cathepsins and glycolytic enzymes including heparanase and hyaluronidases. Matrix proteases evoke epithelial-to-mesenchymal transition (EMT) and regulate ECM turnover under normal procedures as well as cancer cell phenotype, motility, invasion, autophagy, angiogenesis and exosome formation through vital signaling cascades. ECM remodeling is also achieved by glycolytic enzymes that are essential for cancer cell survival, proliferation and tumor progression. In this article, the types of major matrix remodeling enzymes, their effects in cancer initiation, propagation and progression as well as their pharmacological targeting and ongoing clinical trials are presented and critically discussed.
Keywords: cancer; cathepsins; extracellular matrix; heparanase; hyaluronidases; matrix metalloproteinases; plasminogen activators.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

