Hesperidin Promotes Osteogenesis and Modulates Collagen Matrix Organization and Mineralization In Vitro and In Vivo
- PMID: 33810030
- PMCID: PMC8004833
- DOI: 10.3390/ijms22063223
Hesperidin Promotes Osteogenesis and Modulates Collagen Matrix Organization and Mineralization In Vitro and In Vivo
Abstract
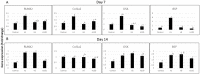
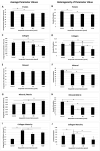
This study evaluated the direct effect of a phytochemical, hesperidin, on pre-osteoblast cell function as well as osteogenesis and collagen matrix quality, as there is little known about hesperidin's influence in mineralized tissue formation and regeneration. Hesperidin was added to a culture of MC3T3-E1 cells at various concentrations. Cell proliferation, viability, osteogenic gene expression and deposited collagen matrix analyses were performed. Treatment with hesperidin showed significant upregulation of osteogenic markers, particularly with lower doses. Mature and compact collagen fibrils in hesperidin-treated cultures were observed by picrosirius red staining (PSR), although a thinner matrix layer was present for the higher dose of hesperidin compared to osteogenic media alone. Fourier-transform infrared spectroscopy indicated a better mineral-to-matrix ratio and matrix distribution in cultures exposed to hesperidin and confirmed less collagen deposited with the 100-µM dose of hesperidin. In vivo, hesperidin combined with a suboptimal dose of bone morphogenetic protein 2 (BMP2) (dose unable to promote healing of a rat mandible critical-sized bone defect) in a collagenous scaffold promoted a well-controlled (not ectopic) pattern of bone formation as compared to a large dose of BMP2 (previously defined as optimal in healing the critical-sized defect, although of ectopic nature). PSR staining of newly formed bone demonstrated that hesperidin can promote maturation of bone organic matrix. Our findings show, for the first time, that hesperidin has a modulatory role in mineralized tissue formation via not only osteoblast cell differentiation but also matrix organization and matrix-to-mineral ratio and could be a potential adjunct in regenerative bone therapies.
Keywords: bone; bone morphogenetic protein; collagen; critical-sized defect; extracellular matrix; hesperidin; osteogenesis; regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Mitigation of BMP-induced inflammation in craniofacial bone regeneration and improvement of bone parameters by dietary hesperidin.Sci Rep. 2024 Jan 31;14(1):2602. doi: 10.1038/s41598-024-52566-7. Sci Rep. 2024. PMID: 38297106 Free PMC article.
-
Normal matrix mineralization induced by strontium ranelate in MC3T3-E1 osteogenic cells.Metabolism. 2004 Apr;53(4):532-7. doi: 10.1016/j.metabol.2003.10.022. Metabolism. 2004. PMID: 15045704
-
Bone morphogenetic protein-2 restores mineralization in glucocorticoid-inhibited MC3T3-E1 osteoblast cultures.J Bone Miner Res. 2003 Jul;18(7):1186-97. doi: 10.1359/jbmr.2003.18.7.1186. J Bone Miner Res. 2003. PMID: 12854828
-
Coupling of Intracellular Calcium Homeostasis and Formation and Secretion of Matrix Vesicles: Their Role in the Mechanism of Biomineralization.Cells. 2025 May 17;14(10):733. doi: 10.3390/cells14100733. Cells. 2025. PMID: 40422236 Free PMC article. Review.
-
The Regulation of Collagen Processing by miRNAs in Disease and Possible Implications for Bone Turnover.Int J Mol Sci. 2021 Dec 22;23(1):91. doi: 10.3390/ijms23010091. Int J Mol Sci. 2021. PMID: 35008515 Free PMC article. Review.
Cited by
-
Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations.Open Life Sci. 2025 Jun 17;20(1):20221032. doi: 10.1515/biol-2022-1032. eCollection 2025. Open Life Sci. 2025. PMID: 40575730 Free PMC article.
-
Mitigation of BMP-induced inflammation in craniofacial bone regeneration and improvement of bone parameters by dietary hesperidin.Sci Rep. 2024 Jan 31;14(1):2602. doi: 10.1038/s41598-024-52566-7. Sci Rep. 2024. PMID: 38297106 Free PMC article.
-
Hesperidin Ameliorates Dexamethasone-Induced Osteoporosis by Inhibiting p53.Front Cell Dev Biol. 2022 Apr 11;10:820922. doi: 10.3389/fcell.2022.820922. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35478958 Free PMC article.
-
Bioactivity, Molecular Mechanism, and Targeted Delivery of Flavonoids for Bone Loss.Nutrients. 2023 Feb 12;15(4):919. doi: 10.3390/nu15040919. Nutrients. 2023. PMID: 36839278 Free PMC article. Review.
-
Editorial: Tissue Stem Cells During Trauma: From Basic Biology to Translational Medicine.Front Cell Dev Biol. 2022 May 26;10:914582. doi: 10.3389/fcell.2022.914582. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35693930 Free PMC article. No abstract available.
References
-
- Kao D.W.K., Kubota A., Nevins M., Fiorellini J.P. The negative effect of combining rhBMP-2 and Bio-Oss on bone formation for maxillary sinus augmentation. Int. J. Periodontics Restor. Dent. 2012;32:61–67. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

