Low Adenovirus Vaccine Doses Administered to Skin Using Microneedle Patches Induce Better Functional Antibody Immunogenicity as Compared to Systemic Injection
- PMID: 33810085
- PMCID: PMC8005075
- DOI: 10.3390/vaccines9030299
Low Adenovirus Vaccine Doses Administered to Skin Using Microneedle Patches Induce Better Functional Antibody Immunogenicity as Compared to Systemic Injection
Abstract
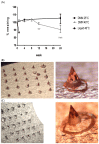
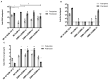
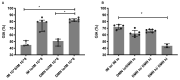
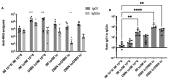
Adenovirus-based vaccines are demonstrating promising clinical potential for multiple infectious diseases, including COVID-19. However, the immunogenicity of the vector itself decreases its effectiveness as a boosting vaccine due to the induction of strong anti-vector neutralizing immunity. Here we determined how dissolvable microneedle patches (DMN) for skin immunization can overcome this issue, using a clinically-relevant adenovirus-based Plasmodium falciparum malaria vaccine, AdHu5-PfRH5, in mice. Incorporation of vaccine into patches significantly enhanced its thermostability compared to the liquid form. Conventional high dose repeated immunization by the intramuscular (IM) route induced low antigen-specific IgG titres and high anti-vector immunity. A low priming dose of vaccine, by the IM route, but more so using DMN patches, induced the most efficacious immune responses, assessed by parasite growth inhibitory activity (GIA) assays. Administration of low dose AdHu5-PfRH5 using patches to the skin, boosted by high dose IM, induced the highest antigen-specific serum IgG response after boosting, the greatest skewing of the antibody response towards the antigen and away from the vector, and the highest efficacy. This study therefore demonstrates that repeated use of the same adenovirus vaccine can be highly immunogenic towards the transgene if a low dose is used to prime the response. It also provides a method of stabilizing adenovirus vaccine, in easy-to-administer dissolvable microneedle patches, permitting storage and distribution out of cold chain.
Keywords: adenovirus; anti-vector immunity; antibody; dose; microneedle; skin; stability; virus vector vaccine.
Conflict of interest statement
O.F. and A.C.M. are inventors of patents that have been or may be licensed to companies developing microneedle-based products. This potential competing interest has been disclosed and is being managed by University College Cork. S.J.D. is a named inventor on patent applications relating to RH5 and/or adenovirus-based vaccines and immunization regimens. All other authors declare that they have no known competing financial interests. None of the authors have personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Microneedle-mediated immunization of an adenovirus-based malaria vaccine enhances antigen-specific antibody immunity and reduces anti-vector responses compared to the intradermal route.Sci Rep. 2014 Aug 21;4:6154. doi: 10.1038/srep06154. Sci Rep. 2014. PMID: 25142082 Free PMC article.
-
Induction of broad immunity by thermostabilised vaccines incorporated in dissolvable microneedles using novel fabrication methods.J Control Release. 2016 Mar 10;225:192-204. doi: 10.1016/j.jconrel.2016.01.019. Epub 2016 Jan 14. J Control Release. 2016. PMID: 26774221
-
A TLR9-adjuvanted vaccine formulated into dissolvable microneedle patches or cationic liposomes protects against leishmaniasis after skin or subcutaneous immunization.Int J Pharm. 2020 Aug 30;586:119390. doi: 10.1016/j.ijpharm.2020.119390. Epub 2020 Jun 12. Int J Pharm. 2020. PMID: 32540349
-
Microneedle Patches as Drug and Vaccine Delivery Platform.Curr Med Chem. 2017;24(22):2413-2422. doi: 10.2174/0929867324666170526124053. Curr Med Chem. 2017. PMID: 28552053 Review.
-
Microneedle patches for vaccination in developing countries.J Control Release. 2016 Oct 28;240:135-141. doi: 10.1016/j.jconrel.2015.11.019. Epub 2015 Nov 18. J Control Release. 2016. PMID: 26603347 Free PMC article. Review.
Cited by
-
Microneedles: An Emerging Vaccine Delivery Tool and a Prospective Solution to the Challenges of SARS-CoV-2 Mass Vaccination.Pharmaceutics. 2023 Apr 27;15(5):1349. doi: 10.3390/pharmaceutics15051349. Pharmaceutics. 2023. PMID: 37242591 Free PMC article. Review.
-
Buccal and Sublingual Vaccines: A Review on Oral Mucosal Immunization and Delivery Systems.Vaccines (Basel). 2021 Oct 14;9(10):1177. doi: 10.3390/vaccines9101177. Vaccines (Basel). 2021. PMID: 34696284 Free PMC article. Review.
-
Microneedle arrays integrated with living organisms for smart biomedical applications.Theranostics. 2021 Oct 25;11(20):10012-10029. doi: 10.7150/thno.66478. eCollection 2021. Theranostics. 2021. PMID: 34815801 Free PMC article. Review.
-
Exploiting Unique Features of Microneedles to Modulate Immunity.Adv Mater. 2023 Dec;35(52):e2302410. doi: 10.1002/adma.202302410. Epub 2023 Nov 5. Adv Mater. 2023. PMID: 37380199 Free PMC article. Review.
-
Dissolving microneedles for nucleic acid delivery: A systematic search, review, and data synthesis.Acta Biomater. 2025 Jun 15;200:115-131. doi: 10.1016/j.actbio.2025.05.025. Epub 2025 May 9. Acta Biomater. 2025. PMID: 40349901 Review.
References
-
- Zhu F.C., Guan X.H., Li Y.H., Huang J.Y., Jiang T., Hou L.H., Li J.X., Yang B.F., Wang L., Wang W.J., et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. - DOI - PMC - PubMed
-
- Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., Voysey M., Aley P.K., Angus B., Babbage G., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. - DOI - PMC - PubMed
-
- Wolfson L.J., Gasse F., Lee-Martin S.P., Lydon P., Magan A., Tibouti A., Johns B., Hutubessy R., Salama P., Okwo-Bele J.M. Estimating the costs of achieving the WHO-UNICEF Global Immunization Vision and Strategy, 2006–2015. Bull. World Health Organ. 2008;86:27–39. doi: 10.2471/BLT.07.045096. - DOI - PMC - PubMed
-
- Payne R.O., Silk S.E., Elias S.C., Miura K., Diouf A., Galaway F., de Graaf H., Brendish N.J., Poulton I.D., Griffiths O.J., et al. Human vaccination against RH5 induces neutralizing antimalarial antibodies that inhibit RH5 invasion complex interactions. JCI Insight. 2017;2:e96381. doi: 10.1172/jci.insight.96381. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

