Solution and Solid State Studies of Urea Derivatives of DITIPIRAM Acting as Powerful Anion Receptors
- PMID: 33810117
- PMCID: PMC8004752
- DOI: 10.3390/molecules26061788
Solution and Solid State Studies of Urea Derivatives of DITIPIRAM Acting as Powerful Anion Receptors
Abstract
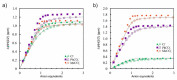
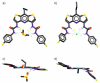
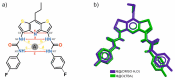
Herein, we present the synthesis and anion binding studies of a family of homologous molecular receptors 4-7 based on a DITIPIRAM (8-propyldithieno-[3,2-b:2',3'-e]-pyridine-3,5-di-amine) platform decorated with various urea para-phenyl substituents (NO2, F, CF3, and Me). Solution, X-ray, and DFT studies reveal that the presented host-guest system offers a convergent array of four urea NH hydrogen bond donors to anions allowing the formation of remarkably stable complexes with carboxylates (acetate, benzoate) and chloride anions in solution, even in competitive solvent mixtures such as DMSO-d6/H2O 99.5/0.5 (v/v) and DMSO-d3/MeOH-d3 9:1 (v/v). The most effective derivatives among the series turned out to be receptors 5 and 6 containing electron-withdrawing F- and -CF3para-substituents, respectively.
Keywords: DITIPIRAM; acyclic receptors; anion recognition; supramolecular chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Amendola V., Bonizzoni M., Esteban-Gómez D., Fabbrizzi L., Licchelli M., Sancenón F., Taglietti A. Some guidelines for the design of anion receptors. Coord. Chem. Rev. 2006;250:1451–1470. doi: 10.1016/j.ccr.2006.01.006. - DOI
-
- Haridas V., Sahu S., Kumar P.P.P., Sapala A.R. Triazole: A new motif for anion recognition. RSC Adv. 2012;2:12594–12605. doi: 10.1039/c2ra21497k. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

