A Novel Microfluidic Device for Blood Plasma Filtration
- PMID: 33810143
- PMCID: PMC8004888
- DOI: 10.3390/mi12030336
A Novel Microfluidic Device for Blood Plasma Filtration
Abstract
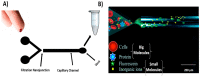
The use of whole blood and some biological specimens, such as urine, saliva, and seminal fluid are limited in clinical laboratory analysis due to the interference of proteins with other small molecules in the matrix and blood cells with optical detection methods. Previously, we developed a microfluidic device featuring an electrokinetic size and mobility trap (SMT) for on-chip extract, concentrate, and separate small molecules from a biological sample like whole blood. The device was used to on-chip filtrate the whole blood from the blood cells and plasma proteins and then on-chip extract and separate the aminoglycoside antibiotic drugs within 3 min. Herein, a novel microfluidic device featuring a nano-junction similar to those reported in the previous work formed by dielectric breakdown was developed for on-chip filtration and out-chip collection of blood plasma with a high extraction yield of 62% within less than 5 min. The filtered plasma was analyzed using our previous device to show the ability of this new device to remove blood cells and plasma proteins. The filtration device shows a high yield of plasma allowing it to detect a low concentration of analytes from the whole blood.
Keywords: blood molecules; blood plasma filtration; chip extract; microfluidics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources

