Current FDA-Approved Therapies for High-Grade Malignant Gliomas
- PMID: 33810154
- PMCID: PMC8004675
- DOI: 10.3390/biomedicines9030324
Current FDA-Approved Therapies for High-Grade Malignant Gliomas
Abstract
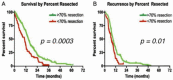
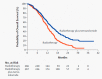
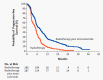
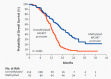
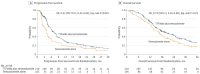
The standard of care (SOC) for high-grade gliomas (HGG) is maximally safe surgical resection, followed by concurrent radiation therapy (RT) and temozolomide (TMZ) for 6 weeks, then adjuvant TMZ for 6 months. Before this SOC was established, glioblastoma (GBM) patients typically lived for less than one year after diagnosis, and no adjuvant chemotherapy had demonstrated significant survival benefits compared with radiation alone. In 2005, the Stupp et al. randomized controlled trial (RCT) on newly diagnosed GBM patients concluded that RT plus TMZ compared to RT alone significantly improved overall survival (OS) (14.6 vs. 12.1 months) and progression-free survival (PFS) at 6 months (PFS6) (53.9% vs. 36.4%). Outside of TMZ, there are four drugs and one device FDA-approved for the treatment of HGGs: lomustine, intravenous carmustine, carmustine wafer implants, bevacizumab (BVZ), and tumor treatment fields (TTFields). These treatments are now mainly used to treat recurrent HGGs and symptoms. TTFields is the only treatment that has been shown to improve OS (20.5 vs. 15.6 months) and PFS6 (56% vs. 37%) in comparison to the current SOC. TTFields is the newest addition to this list of FDA-approved treatments, but has not been universally accepted yet as part of SOC.
Keywords: FDA-approved; bevacizumab; carmustine; glioblastoma; high-grade glioma; lomustine; malignant glioma; standard of care; temozolomide; tumor treatment fields.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ostrom Q.T., Gittleman H., Liao P., Rouse C., Chen Y., Dowling J., Wolinsky Y., Kruchko C., Barnholtz-Sloan J. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16:iv1–iv63. doi: 10.1093/neuonc/nou223. - DOI - PMC - PubMed
-
- Survival Rates for Selected Adult Brain and Spinal Cord Tumors. [(accessed on 30 September 2020)]; Available online: https://www.cancer.org/cancer/brain-spinal-cord-tumors-adults/detection-....
-
- Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
-
- Fernandes C., Costa A., Osório L., Lago R.C., Linhares P., Carvalho B., Caeiro C. Current standards of care in glioblastoma therapy. In: De Vleeschouwer S., editor. Glioblastoma. Codon Publications; Brisbane, Australia: 2017. Chapter 11. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

