PET Diagnostic Molecules Utilizing Multimeric Cyclic RGD Peptide Analogs for Imaging Integrin αvβ3 Receptors
- PMID: 33810198
- PMCID: PMC8005094
- DOI: 10.3390/molecules26061792
PET Diagnostic Molecules Utilizing Multimeric Cyclic RGD Peptide Analogs for Imaging Integrin αvβ3 Receptors
Abstract
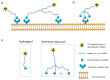
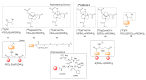
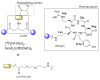
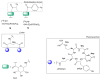
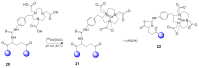
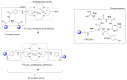
Multimeric ligands consisting of multiple pharmacophores connected to a single backbone have been widely investigated for diagnostic and therapeutic applications. In this review, we summarize recent developments regarding multimeric radioligands targeting integrin αvβ3 receptors on cancer cells for molecular imaging and diagnostic applications using positron emission tomography (PET). Integrin αvβ3 receptors are glycoproteins expressed on the cell surface, which have a significant role in tumor angiogenesis. They act as receptors for several extracellular matrix proteins exposing the tripeptide sequence arginine-glycine-aspartic (RGD). Cyclic RDG peptidic ligands c(RGD) have been developed for integrin αvβ3 tumor-targeting positron emission tomography (PET) diagnosis. Several c(RGD) pharmacophores, connected with the linker and conjugated to a chelator or precursor for radiolabeling with different PET radionuclides (18F, 64Cu, and 68Ga), have resulted in multimeric ligands superior to c(RGD) monomers. The binding avidity, pharmacodynamic, and PET imaging properties of these multimeric c(RGD) radioligands, in relation to their structural characteristics are analyzed and discussed. Furthermore, specific examples from preclinical studies and clinical investigations are included.
Keywords: PET imaging; cyclic RGD; integrin αvβ3; multimeric radioligands.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Yim C.-B., Van Der Wildt B., Dijkgraaf I., Joosten L., Eek A., Versluis C., Rijkers D.T.S., Boerman O.C., Liskamp R.M.J. Spacer Effects on in vivo Properties of DOTA-Conjugated Dimeric [Tyr3]Octreotate Peptides Synthesized by a “CuI-Click” and “Sulfo-Click” Ligation Method. ChemBioChem. 2011;12:750–760. doi: 10.1002/cbic.201000639. - DOI - PubMed
-
- Baranski A.-C., Schäfer M., Bauder-Wüst U., Wacker A., Schmidt J., Liolios C., Mier W., Haberkorn U., Eisenhut M., Kopka K., et al. Improving the Imaging Contrast of 68Ga-PSMA-11 by Targeted Linker Design: Charged Spacer Moieties Enhance the Pharmacokinetic Properties. Bioconjugate Chem. 2017;28:2485–2492. doi: 10.1021/acs.bioconjchem.7b00458. - DOI - PubMed
-
- Liolios C.C., Fragogeorgi E.A., Zikos C., Loudos G., Xanthopoulos S., Bouziotis P., Paravatou-Petsotas M., Livaniou E., Varvarigou A.D., Sivolapenko G.B. Structural modifications of 99mTc-labelled bombesin-like peptides for optimizing pharmacokinetics in prostate tumor targeting. Int. J. Pharm. 2012;430:1–17. doi: 10.1016/j.ijpharm.2012.02.049. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

