Endocrine-Based Treatments in Clinically-Relevant Subgroups of Hormone Receptor-Positive/HER2-Negative Metastatic Breast Cancer: Systematic Review and Meta-Analysis
- PMID: 33810205
- PMCID: PMC8004645
- DOI: 10.3390/cancers13061458
Endocrine-Based Treatments in Clinically-Relevant Subgroups of Hormone Receptor-Positive/HER2-Negative Metastatic Breast Cancer: Systematic Review and Meta-Analysis
Abstract
A precise assessment of the efficacy of first-/second-line endocrine therapies (ET) ± target therapies (TT) in clinically-relevant subgroups of hormone receptor-positive (HR+)/HER2-negative metastatic breast cancer (MBC) has not yet been conducted. To improve our current knowledge and support clinical decision-making, we thus conducted a systematic literature search to identify all first-/second-line phase II/III randomized clinical trials (RCT) of currently approved or most promising ET ± TT. Then, we performed a meta-analysis to assess progression-free (PFS) and/or overall survival (OS) benefit in several clinically-relevant prespecified subgroups. Thirty-five RCT were included (17,595 patients). Pooled results show significant reductions in the risk of relapse or death of 26-41% and 12-27%, respectively, depending on the clinical subgroup. Combination strategies proved to be more effective than single-agent ET (PFS hazard ratio (HR) range for combinations: 0.60-0.65 vs. HR range for single agent ET: 0.59-1.37; OS HR range for combinations: 0.74-0.87 vs. HR range for single agent ET: 0.68-0.98), with CDK4/6-inhibitors(i) + ET being the most effective regimen. Single agent ET showed comparable efficacy with ET+TT combinations in non-visceral (p = 0.63) and endocrine sensitive disease (p = 0.79), while mTORi-based combinations proved to be a valid therapeutic option in endocrine-resistant tumors, as well as PI3Ki + ET in PIK3CA-mutant tumors. These results strengthen international treatment guidelines and can aid therapeutic decision-making.
Keywords: endocrine therapy; hormone receptor; meta-analysis; metastatic breast cancer; systematic review.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. Any views, opinions, findings, conclusions, or recommendations expressed in this material are those solely of the author(s) and do not necessarily reflect those of the funders. Outside of the submitted work, Mario Giuliano and Sabino De Placido have declared honoraria from Roche, Pfizer, Astra-Zeneca, Novartis, Celgene, Eli Lilly, Amgen and Eisai. Aleix Prat has declared an immediate family member being employed by Novartis, personal honoraria from Pfizer, Novartis, Roche, MSD Oncology, Lilly and Daiichi Sankyo; travel, accommodations and expenses paid by Daiichi Sankyo; research funding from Roche and Novartis; consulting/advisory role for NanoString Technologies, Amgen, Roche, Novartis, Pfizer and Bristol-Myers Squibb; and patent PCT/EP2016/080056: HER2 AS A PREDICTOR OF RESPONSE TO DUAL HER2 BLOCKADE IN THE ABSENCE OF CYTOTOXIC THERAPY. Guy Jerusalem has reported grants, personal fees and non-financial support from Novartis; grants, personal fees and non-financial support from Roche; grants, personal fees and non-financial support from Pfizer; personal fees and non-financial support from Lilly; personal fees and non-financial support from Amgen; personal fees and non-financial support from BMS; personal fees and non-financial support from Astra-Zeneca; personal fees and non-financial support from Daiichi Sankyo; personal fees from Abbvie; non-financial support from Medimmune; and non-financial support from MerckKGaA. Angelo Di Leo has reported advisory board, consultant and honoraria from AZ, Bayer, Celgene, Daiichi-Sankyo, Eisai, Genomic Health, Genentech, Ipsen, Lilly, Novartis, Puma Biotechnology, Pfizer and Roche. Lucia Del Mastro has declared honoraria from Roche, Pfizer, Ipsen, Eli Lilly, Eisai, Novartis, Takeda and MSD; consulting/advisory role for Roche and Eli Lilly; and travel, accommodation and expenses from Roche, Pfizer and Celgene. Fabio Puglisi has declared honoraria for advisory boards; activities as a speaker from Amgen, Astra-Zeneca, Celgene, Eisai, Eli Lilly, Ipsen, MSD, Novartis, Pierre-Fabre, Pfizer, Roche and Takeda; travel grants from Celgene and Roche; and research funding from Astra-Zeneca, Eisai, Roche and Italian Ministry of Health. PierFranco Conte has declared consultant role for Novartis, Eli Lilly, Astra Zeneca and Tesaro; honoraria from BMS, Roche, Eli Lilly, Novartis and AstraZeneca; and research funding from Novartis, Roche, BMS, Merck-KGa, Italian Ministry of Health, Veneto Secretary of Health and University of Padova. Rachel Schiff has declared research funding from AstraZeneca, GlaxoSmithKline, Gilead Sciences, Eli Lilly, and PUMA Biotechnology and consulting/advisory role with compensation for Macrogenics and Eli Lilly. C. Kent Osborne has declared research funding from AstraZeneca and GlaxoSmithKline; advisory boards for Tolmar Pharmaceuticals, Genentech, and AstraZeneca; DMC for Eli Lilly; and stockholder of GeneTex. Mothaffar F. Rimawi has declared research funding from GlaxoSmithKline and Genentech. Lajos Pusztai has received consulting fees and honoraria from Seagen, Pfizer, Astra Zeneca, Merck, Novartis, Bristol-Myers Squibb, Pfizer, Genentech, Eisai, Pieris, Immunomedics, Clovis, Syndax, H3Bio, Radius Health, and Daiichi and institutional research funding from Seagen, AstraZeneca, Merck, Pfizer and Bristol Myers Squibb. Daniele Generali has declared consulting fees from Novartis, Lilly and Pfizer and research funding from LILT, Novartis Astra-Zeneca and University of Trieste. The other authors have nothing to declare.
Figures
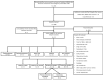




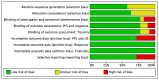
References
-
- Cristofanilli M., Turner N.C., Bondarenko I., Ro J., Im S.-A., Masuda N., Colleoni M., DeMichele A., Loi S., Verma S., et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/s1470-2045(15)00613-0. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

