MicroRNAs, Long Non-Coding RNAs, and Circular RNAs: Potential Biomarkers and Therapeutic Targets in Pheochromocytoma/Paraganglioma
- PMID: 33810219
- PMCID: PMC8036642
- DOI: 10.3390/cancers13071522
MicroRNAs, Long Non-Coding RNAs, and Circular RNAs: Potential Biomarkers and Therapeutic Targets in Pheochromocytoma/Paraganglioma
Abstract
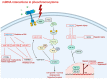
Around 40% of pheochromocytomas/paragangliomas (PPGL) harbor germline mutations, representing the highest heritability among human tumors. All PPGL have metastatic potential, but metastatic PPGL is overall rare. There is no available molecular marker for the metastatic potential of these tumors, and the diagnosis of metastatic PPGL can only be established if metastases are found at "extra-chromaffin" sites. In the era of precision medicine with individually targeted therapies and advanced care of patients, the treatment options for metastatic pheochromocytoma/paraganglioma are still limited. With this review we would like to nurture the idea of the quest for non-coding ribonucleic acids as an area to be further investigated in tumor biology. Non-coding RNA molecules encompassing microRNAs, long non-coding RNAs, and circular RNAs have been implicated in the pathogenesis of various tumors, and were also proposed as valuable diagnostic, prognostic factors, and even potential treatment targets. Given the fact that the pathogenesis of tumors including pheochromocytomas/paragangliomas is linked to epigenetic dysregulation, it is reasonable to conduct studies related to their epigenetic expression profiles and in this brief review we present a synopsis of currently available findings on the relevance of these molecules in these tumors highlighting their diagnostic potential.
Keywords: biomarker; genetics; malignancy; non-coding RNA; paraganglioma; pheochromocytoma; treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The Role of the Metabolome and Non-Coding RNA on Pheochromocytomas and Paragangliomas: An Update.Metabolites. 2022 Feb 1;12(2):131. doi: 10.3390/metabo12020131. Metabolites. 2022. PMID: 35208206 Free PMC article.
-
Pheochromocytoma and paraganglioma: molecular testing and personalized medicine.Curr Opin Oncol. 2016 Jan;28(1):5-10. doi: 10.1097/CCO.0000000000000249. Curr Opin Oncol. 2016. PMID: 26599293 Review.
-
Integrative multi-omics analysis identifies a prognostic miRNA signature and a targetable miR-21-3p/TSC2/mTOR axis in metastatic pheochromocytoma/paraganglioma.Theranostics. 2019 Jul 9;9(17):4946-4958. doi: 10.7150/thno.35458. eCollection 2019. Theranostics. 2019. PMID: 31410193 Free PMC article.
-
Transcriptome Analysis of lncRNAs in Pheochromocytomas and Paragangliomas.J Clin Endocrinol Metab. 2020 Mar 1;105(3):dgz168. doi: 10.1210/clinem/dgz168. J Clin Endocrinol Metab. 2020. PMID: 31678991
-
The systems of metastatic potential prediction in pheochromocytoma and paraganglioma.Am J Cancer Res. 2020 Mar 1;10(3):769-780. eCollection 2020. Am J Cancer Res. 2020. PMID: 32266090 Free PMC article. Review.
Cited by
-
New Insights in the Genetics and Genomics of Adrenocortical Tumors and Pheochromocytomas.Cancers (Basel). 2022 Feb 21;14(4):1094. doi: 10.3390/cancers14041094. Cancers (Basel). 2022. PMID: 35205841 Free PMC article.
-
Hypoxia signaling pathway: A central mediator in endocrine tumors.Front Endocrinol (Lausanne). 2023 Jan 9;13:1103075. doi: 10.3389/fendo.2022.1103075. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36699028 Free PMC article. Review.
-
The Role of the Metabolome and Non-Coding RNA on Pheochromocytomas and Paragangliomas: An Update.Metabolites. 2022 Feb 1;12(2):131. doi: 10.3390/metabo12020131. Metabolites. 2022. PMID: 35208206 Free PMC article.
-
The Emerging Role of Long Non-Coding RNAs in Esophageal Cancer: Functions in Tumorigenesis and Clinical Implications.Front Pharmacol. 2022 May 13;13:885075. doi: 10.3389/fphar.2022.885075. eCollection 2022. Front Pharmacol. 2022. PMID: 35645836 Free PMC article. Review.
-
CircTP63 promotes cell proliferation and invasion by regulating EZH2 via sponging miR-217 in gallbladder cancer.Cancer Cell Int. 2021 Nov 17;21(1):608. doi: 10.1186/s12935-021-02316-w. Cancer Cell Int. 2021. PMID: 34789260 Free PMC article.
References
-
- Beard C.M., Sheps S.G., Kurland L.T., Carney J.A., Lie J.T. Occurrence of pheochromocytoma in Rochester, Minnesota, 1950 through 1979. Mayo Clin. Proc. 1983;58:802–804. - PubMed
-
- Pereira B.D., Luiz H.V., Ferreira A.G., Portugal J. Paraganglioma: A Multidisciplinary Approach. Codon Publications; Brisbane, Australia: 2019. Genetics of Pheochromocytoma and Paraganglioma; pp. 1–22. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

