A Simple, Fast and Portable Method for Electrochemical Detection of Adenine Released by Ricin Enzymatic Activity
- PMID: 33810228
- PMCID: PMC8066795
- DOI: 10.3390/toxins13040238
A Simple, Fast and Portable Method for Electrochemical Detection of Adenine Released by Ricin Enzymatic Activity
Abstract
International authorities classify ricin toxin present in castor seed as a potential agent for use in bioterrorism. Therefore, the detection, identification, and characterization of ricin in various sample matrices are considered necessary actions for risk assessment during a suspected exposure. This study reports a portable electrochemical assay for detecting active ricin based on the adenine electro-oxidation released from herring sperm DNA substrate by its catalytic action. Also, kinetic parameters were calculated, and the values were Km of 3.14 µM and Kcat 2107 min-1. A linear response was found in optimized experimental conditions for ricin concentrations ranging from 8 to 120 ng/mL, and with a detection limit of 5.14 ng/mL. This proposed detection strategy emphasizes the possibility of field detection of active ricin in food matrices and can be applied to other endonucleolytic activities.
Keywords: depurination reaction; herring sperm DNA; kinetic analysis; ricin; square wave voltammetry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

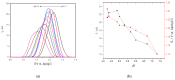
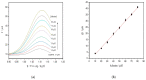

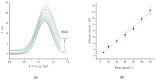
Similar articles
-
An Electrochemical Approach to Follow and Evaluate the Kinetic Catalysis of Ricin on hsDNA.Life (Basel). 2021 Apr 29;11(5):405. doi: 10.3390/life11050405. Life (Basel). 2021. PMID: 33946642 Free PMC article.
-
Detecting ricin: sensitive luminescent assay for ricin A-chain ribosome depurination kinetics.Anal Chem. 2009 Apr 15;81(8):2847-53. doi: 10.1021/ac8026433. Anal Chem. 2009. PMID: 19364139 Free PMC article.
-
Ricin A-chain: kinetics, mechanism, and RNA stem-loop inhibitors.Biochemistry. 1998 Aug 18;37(33):11605-13. doi: 10.1021/bi980990p. Biochemistry. 1998. PMID: 9708998
-
Ricin detection: tracking active toxin.Biotechnol Adv. 2015 Jan-Feb;33(1):117-123. doi: 10.1016/j.biotechadv.2014.11.012. Epub 2014 Dec 4. Biotechnol Adv. 2015. PMID: 25481398 Review.
-
Ricin. Mechanisms of cytotoxicity.Toxicol Rev. 2003;22(1):53-64. doi: 10.2165/00139709-200322010-00006. Toxicol Rev. 2003. PMID: 14579547 Review.
Cited by
-
Functional magnetic nanoparticle-based affinity probe for MALDI mass spectrometric detection of ricin B.Mikrochim Acta. 2021 Sep 12;188(10):339. doi: 10.1007/s00604-021-04991-y. Mikrochim Acta. 2021. PMID: 34510288
-
Isomorphic Fluorescent Nucleosides Facilitate Real-Time Monitoring of RNA Depurination by Ribosome Inactivating Proteins.Chemistry. 2022 Jun 21;28(35):e202200994. doi: 10.1002/chem.202200994. Epub 2022 May 12. Chemistry. 2022. PMID: 35390188 Free PMC article.
-
A Self-Driven Microfluidic Chip for Ricin and Abrin Detection.Sensors (Basel). 2022 May 2;22(9):3461. doi: 10.3390/s22093461. Sensors (Basel). 2022. PMID: 35591151 Free PMC article.
-
Establishment and Comparison of Detection Methods for Ricin and Abrin Based on Their Depurination Activities.Toxins (Basel). 2025 Apr 3;17(4):177. doi: 10.3390/toxins17040177. Toxins (Basel). 2025. PMID: 40278675 Free PMC article.
-
Innovative Ricin Toxin Detection: Unraveling Apurinic/Apyrimidinic Lyase Activity and Developing Fluorescence Sensors.Anal Chem. 2025 Feb 18;97(6):3608-3616. doi: 10.1021/acs.analchem.4c06016. Epub 2025 Jan 22. Anal Chem. 2025. PMID: 39843920 Free PMC article.
References
-
- Endo Y., Mitsui K., Motizuki M., Tsurugi K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J. Biol. Chem. 1987;262:5908–5912. doi: 10.1016/S0021-9258(18)45660-8. - DOI - PubMed
-
- Walsh T.A., Morgan A.E., Hey T.D. Characterization and molecular cloning of a proenzyme form of a ribosome-inactivating protein from maize. Novel mechanism of proenzyme activation by proteolytic removal of a 2.8-kilodalton internal peptide segment. J. Biol. Chem. 1991;266:23422–23427. doi: 10.1016/S0021-9258(18)54513-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

