Deletion of Trpm4 Alters the Function of the Nav1.5 Channel in Murine Cardiac Myocytes
- PMID: 33810249
- PMCID: PMC8037196
- DOI: 10.3390/ijms22073401
Deletion of Trpm4 Alters the Function of the Nav1.5 Channel in Murine Cardiac Myocytes
Abstract
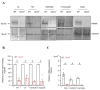
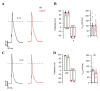
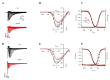
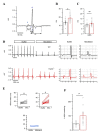
Transient receptor potential melastatin member 4 (TRPM4) encodes a Ca2+-activated, non-selective cation channel that is functionally expressed in several tissues, including the heart. Pathogenic mutants in TRPM4 have been reported in patients with inherited cardiac diseases, including conduction blockage and Brugada syndrome. Heterologous expression of mutant channels in cell lines indicates that these mutations can lead to an increase or decrease in TRPM4 expression and function at the cell surface. While the expression and clinical variant studies further stress the importance of TRPM4 in cardiac function, the cardiac electrophysiological phenotypes in Trpm4 knockdown mouse models remain incompletely characterized. To study the functional consequences of Trpm4 deletion on cardiac electrical activity in mice, we performed perforated-patch clamp and immunoblotting studies on isolated atrial and ventricular cardiac myocytes and surfaces, as well as on pseudo- and intracardiac ECGs, either in vivo or in Langendorff-perfused explanted mouse hearts. We observed that TRPM4 is expressed in atrial and ventricular cardiac myocytes and that deletion of Trpm4 unexpectedly reduces the peak Na+ currents in myocytes. Hearts from Trpm4-/- mice presented increased sensitivity towards mexiletine, a Na+ channel blocker, and slower intraventricular conduction, consistent with the reduction of the peak Na+ current observed in the isolated cardiac myocytes. This study suggests that TRPM4 expression impacts the Na+ current in murine cardiac myocytes and points towards a novel function of TRPM4 regulating the Nav1.5 function in murine cardiac myocytes.
Keywords: SCN5A; TRPM4; cardiac conduction disorder; channelosome; intracardiac ECG; mexiletine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
TRPM4 non-selective cation channels influence action potentials in rabbit Purkinje fibres.J Physiol. 2016 Jan 15;594(2):295-306. doi: 10.1113/JP271347. Epub 2015 Dec 14. J Physiol. 2016. PMID: 26548780 Free PMC article.
-
Shear stress activates monovalent cation channel transient receptor potential melastatin subfamily 4 in rat atrial myocytes via type 2 inositol 1,4,5-trisphosphate receptors and Ca(2+) release.J Physiol. 2016 Jun 1;594(11):2985-3004. doi: 10.1113/JP270887. Epub 2016 Feb 9. J Physiol. 2016. PMID: 26751048 Free PMC article.
-
Uncovering the arrhythmogenic potential of TRPM4 activation in atrial-derived HL-1 cells using novel recording and numerical approaches.Cardiovasc Res. 2017 Aug 1;113(10):1243-1255. doi: 10.1093/cvr/cvx117. Cardiovasc Res. 2017. PMID: 28898995
-
TRPM4 channels in the cardiovascular system.Curr Opin Pharmacol. 2014 Apr;15:68-73. doi: 10.1016/j.coph.2013.12.003. Epub 2013 Dec 25. Curr Opin Pharmacol. 2014. PMID: 24721656 Review.
-
TRPM4 in cardiac electrical activity.Cardiovasc Res. 2015 Oct 1;108(1):21-30. doi: 10.1093/cvr/cvv213. Epub 2015 Aug 13. Cardiovasc Res. 2015. PMID: 26272755 Review.
Cited by
-
Defining the role of TRPM4 in broadly responsive taste receptor cells.Front Cell Neurosci. 2023 Mar 22;17:1148995. doi: 10.3389/fncel.2023.1148995. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37032837 Free PMC article.
-
Electrophysiological Effects of the Transient Receptor Potential Melastatin 4 Channel Inhibitor (4-Chloro-2-(2-chlorophenoxy)acetamido) Benzoic Acid (CBA) in Canine Left Ventricular Cardiomyocytes.Int J Mol Sci. 2021 Aug 31;22(17):9499. doi: 10.3390/ijms22179499. Int J Mol Sci. 2021. PMID: 34502410 Free PMC article.
-
The Role of TRPM4 in Cardiac Electrophysiology and Arrhythmogenesis.Int J Mol Sci. 2023 Jul 22;24(14):11798. doi: 10.3390/ijms241411798. Int J Mol Sci. 2023. PMID: 37511555 Free PMC article. Review.
-
Microtubule plus-end tracking proteins: novel modulators of cardiac sodium channels and arrhythmogenesis.Cardiovasc Res. 2023 Jul 4;119(7):1461-1479. doi: 10.1093/cvr/cvad052. Cardiovasc Res. 2023. PMID: 37040608 Free PMC article.
-
Genetic Basis of Brugada Syndrome.Biomedicines. 2025 Jul 16;13(7):1740. doi: 10.3390/biomedicines13071740. Biomedicines. 2025. PMID: 40722809 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

