Bis-3-Chloropiperidines Targeting TAR RNA as A Novel Strategy to Impair the HIV-1 Nucleocapsid Protein
- PMID: 33810333
- PMCID: PMC8038054
- DOI: 10.3390/molecules26071874
Bis-3-Chloropiperidines Targeting TAR RNA as A Novel Strategy to Impair the HIV-1 Nucleocapsid Protein
Abstract
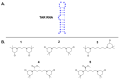
Specific RNA sequences regulate functions essential to life. The Trans-Activation Response element (TAR) is an RNA stem-bulge-loop structure involved in several steps of HIV-1 replication. In this work, we show how RNA targeting can inhibit HIV-1 nucleocapsid (NC), a highly conserved protein known to catalyze nucleic acid melting and strand transfers during reverse transcription. Our RNA targeting strategy consists of the employment of bis-3-chloropiperidines (B-CePs) to impair RNA melting through bifunctional alkylation. Specific interactions between B-CePs and TAR RNA were analytically investigated by gel electrophoresis and mass spectrometry, allowing the elucidation of B-CePs' recognition of TAR, and highlighting an RNA-directed mechanism of protein inhibition. We propose that B-CePs can freeze TAR tridimensional conformation, impairing NC-induced dynamics and finally inhibiting its functions in vitro.
Keywords: TAR-RNA; alkylating agents; bis-3-chloropiperidines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Hermann T. RNA Therapeutics. Topics in Medicinal Chemistry. Volume 27 Springer; Cham, Swithzerland: 2017. Viral RNA Targets and Their Small Molecule Ligands.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

