ACTH(6-9)PGP Peptide Protects SH-SY5Y Cells from H2O2, tert-Butyl Hydroperoxide, and Cyanide Cytotoxicity via Stimulation of Proliferation and Induction of Prosurvival-Related Genes
- PMID: 33810344
- PMCID: PMC8036943
- DOI: 10.3390/molecules26071878
ACTH(6-9)PGP Peptide Protects SH-SY5Y Cells from H2O2, tert-Butyl Hydroperoxide, and Cyanide Cytotoxicity via Stimulation of Proliferation and Induction of Prosurvival-Related Genes
Abstract
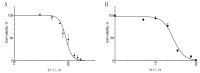
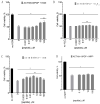
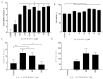
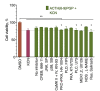
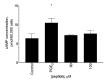
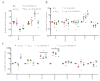
Stabilized melanocortin analog peptide ACTH(6-9)PGP (HFRWPGP) possesses a wide range of neuroprotective activities. However, its mechanism of action remains poorly understood. In this paper, we present a study of the proproliferative and cytoprotective activity of the adrenocorticotropic hormone fragment 6-9 (HFRW) linked with the peptide prolyine-glycyl-proline on the SH-SY5Y cells in the model of oxidative stress-related toxicity. The peptide dose-dependently protected cells from H2O2, tert-butyl hydroperoxide, and KCN and demonstrated proproliferative activity. The mechanism of its action was the modulation of proliferation-related NF-κB genes and stimulation of prosurvival NRF2-gene-related pathway, as well as a decrease in apoptosis.
Keywords: ACTH(6–9); H2O2; MPP+; cyanide; melanocortins; neuroprotection; oxidative stress; tert-butyl hydroperoxide.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Neuroprotective effects of aucubin on hydrogen peroxide-induced toxicity in human neuroblastoma SH-SY5Y cells via the Nrf2/HO-1 pathway.Phytomedicine. 2021 Jul;87:153577. doi: 10.1016/j.phymed.2021.153577. Epub 2021 Apr 18. Phytomedicine. 2021. PMID: 33994055
-
Orexin-A protects SH-SY5Y cells against H2O2-induced oxidative damage via the PI3K/MEK1/2/ERK1/2 signaling pathway.Int J Immunopathol Pharmacol. 2018 Jan-Dec;32:2058738418785739. doi: 10.1177/2058738418785739. Int J Immunopathol Pharmacol. 2018. PMID: 29983082 Free PMC article.
-
Pretreatment of MQA, a caffeoylquinic acid derivative compound, protects against H2O2-induced oxidative stress in SH-SY5Y cells.Neurol Res. 2016 Dec;38(12):1079-1087. doi: 10.1080/01616412.2016.1245030. Epub 2016 Nov 1. Neurol Res. 2016. PMID: 27800716
-
Protective effects of distinct proline-rich oligopeptides from B. jararaca snake venom against oxidative stress-induced neurotoxicity.Toxicon. 2019 Sep;167:29-37. doi: 10.1016/j.toxicon.2019.06.012. Epub 2019 Jun 7. Toxicon. 2019. PMID: 31181294
-
Naringenin Inhibit the Hydrogen Peroxide-Induced SH-SY5Y Cells Injury Through Nrf2/HO-1 Pathway.Neurotox Res. 2019 Nov;36(4):796-805. doi: 10.1007/s12640-019-00046-6. Epub 2019 May 10. Neurotox Res. 2019. PMID: 31076999
Cited by
-
A Red-Berry Mixture as a Nutraceutical: Detailed Composition and Neuronal Protective Effect.Molecules. 2021 May 27;26(11):3210. doi: 10.3390/molecules26113210. Molecules. 2021. PMID: 34071973 Free PMC article.
-
Synthetic Adrenocorticotropic Peptides Modulate the Expression Pattern of Immune Genes in Rat Brain following the Early Post-Stroke Period.Genes (Basel). 2023 Jun 30;14(7):1382. doi: 10.3390/genes14071382. Genes (Basel). 2023. PMID: 37510287 Free PMC article.
-
Neuroprotective Peptides and New Strategies for Ischemic Stroke Drug Discoveries.Genes (Basel). 2023 Apr 22;14(5):953. doi: 10.3390/genes14050953. Genes (Basel). 2023. PMID: 37239313 Free PMC article. Review.
-
Effects of ACTH6-9-Pro-Gly-Pro Peptide on the Levels of Pro- and Anti-Inflammatory Cytokines in Wistar Rats under Conditions of Chronic Restraint Stress.Bull Exp Biol Med. 2023 Apr;174(6):716-718. doi: 10.1007/s10517-023-05777-3. Epub 2023 May 8. Bull Exp Biol Med. 2023. PMID: 37157045
-
Melanocortin Derivatives Induced Vascularization and Neuroglial Proliferation in the Rat Brain under Conditions of Cerebral Ischemia.Curr Issues Mol Biol. 2024 Mar 5;46(3):2071-2092. doi: 10.3390/cimb46030133. Curr Issues Mol Biol. 2024. PMID: 38534749 Free PMC article.
References
-
- Ashmarin I.P., Nezavibat’ko V.N., Myasoedov N.F., Kamenskii A.A., Grivennikov I.A., Ponomareva-Stepnaya M.A., Andreeva L.A., Kaplan A.Y., Koshelev V.B. A Nootropic Analog of Adrenocorticotrophic Hormone 4-10--Semax (Experience of 15 Years of Development and Investigation) Zh. Vyssh. Nerv. Deyat. 1997;47:420–430. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

