PER2 Circadian Oscillation Sensitizes Esophageal Cancer Cells to Chemotherapy
- PMID: 33810377
- PMCID: PMC8065910
- DOI: 10.3390/biology10040266
PER2 Circadian Oscillation Sensitizes Esophageal Cancer Cells to Chemotherapy
Abstract
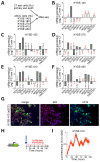
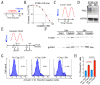
Esophageal squamous cell carcinoma (eSCC) accounts for more than 85% cases of esophageal cancer worldwide and the 5-year survival rate associated with metastatic eSCC is poor. This low survival rate is the consequence of a complex mechanism of resistance to therapy and tumor relapse. To effectively reduce the mortality rate of this disease, we need to better understand the molecular mechanisms underlying the development of resistance to therapy and translate that knowledge into novel approaches for cancer treatment. The circadian clock orchestrates several physiological processes through the establishment and synchronization of circadian rhythms. Since cancer cells need to fuel rapid proliferation and increased metabolic demands, the escape from circadian rhythm is relevant in tumorigenesis. Although clock related genes may be globally repressed in human eSCC samples, PER2 expression still oscillates in some human eSCC cell lines. However, the consequences of this circadian rhythm are still unclear. In the present study, we confirm that PER2 oscillations still occur in human cancer cells in vitro in spite of a deregulated circadian clock gene expression. Profiling of eSCC cells by RNAseq reveals that when PER2 expression is low, several transcripts related to apoptosis are upregulated. Consistently, treating eSCC cells with cisplatin when PER2 expression is low enhances DNA damage and leads to a higher apoptosis rate. Interestingly, this process is conserved in a mouse model of chemically-induced eSCC ex vivo. These results therefore suggest that response to therapy might be enhanced in esophageal cancers using chronotherapy.
Keywords: apoptosis; chemotherapy; chronotherapy; circadian clock; esophagus cancer; squamous cell carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
A role for the clock period circadian regulator 2 gene in regulating the clock gene network in human oral squamous cell carcinoma cells.Oncol Lett. 2018 Apr;15(4):4185-4192. doi: 10.3892/ol.2018.7825. Epub 2018 Jan 19. Oncol Lett. 2018. PMID: 29541184 Free PMC article.
-
Alterations of circadian clockworks during differentiation and apoptosis of rat ovarian cells.Chronobiol Int. 2011 Jul;28(6):477-87. doi: 10.3109/07420528.2011.589933. Chronobiol Int. 2011. PMID: 21797776
-
Modeling the interactions of sense and antisense Period transcripts in the mammalian circadian clock network.PLoS Comput Biol. 2018 Feb 15;14(2):e1005957. doi: 10.1371/journal.pcbi.1005957. eCollection 2018 Feb. PLoS Comput Biol. 2018. PMID: 29447160 Free PMC article.
-
Chronopharmacological strategies focused on chrono-drug discovery.Pharmacol Ther. 2019 Oct;202:72-90. doi: 10.1016/j.pharmthera.2019.05.018. Epub 2019 Jun 5. Pharmacol Ther. 2019. PMID: 31173839 Review.
-
Cisplatin's dual-effect on the circadian clock triggers proliferation and apoptosis.Neurobiol Sleep Circadian Rhythms. 2020 Jul 30;9:100054. doi: 10.1016/j.nbscr.2020.100054. eCollection 2020 Nov. Neurobiol Sleep Circadian Rhythms. 2020. PMID: 33364523 Free PMC article. Review.
Cited by
-
Why does circadian timing of administration matter for immune checkpoint inhibitors' efficacy?Br J Cancer. 2024 Sep;131(5):783-796. doi: 10.1038/s41416-024-02704-9. Epub 2024 Jun 4. Br J Cancer. 2024. PMID: 38834742 Free PMC article. Review.
-
NEK2 Control of Esophageal Squamous Cell Carcinoma Growth Based on Circadian Oscillation.Cancer Sci. 2025 May;116(5):1282-1294. doi: 10.1111/cas.16461. Epub 2025 Feb 18. Cancer Sci. 2025. PMID: 39963019 Free PMC article.
-
Biological clock regulation by the PER gene family: a new perspective on tumor development.Front Cell Dev Biol. 2024 May 15;12:1332506. doi: 10.3389/fcell.2024.1332506. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38813085 Free PMC article. Review.
-
Potential of melatonin to reverse epigenetic aberrations in oral cancer: new findings.EXCLI J. 2023 Dec 12;22:1280-1310. doi: 10.17179/excli2023-6624. eCollection 2023. EXCLI J. 2023. PMID: 38234969 Free PMC article. Review.
-
The role of circadian rhythm regulator PERs in oxidative stress, immunity, and cancer development.Cell Commun Signal. 2025 Jan 16;23(1):30. doi: 10.1186/s12964-025-02040-2. Cell Commun Signal. 2025. PMID: 39825442 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

