Characterization of Natural and Alkaline-Oxidized Proanthocyanidins in Plant Extracts by Ultrahigh-Resolution UHPLC-MS/MS
- PMID: 33810382
- PMCID: PMC8037856
- DOI: 10.3390/molecules26071873
Characterization of Natural and Alkaline-Oxidized Proanthocyanidins in Plant Extracts by Ultrahigh-Resolution UHPLC-MS/MS
Abstract
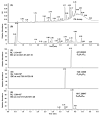
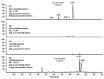
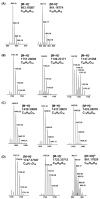
In this study, we analyzed the proanthocyanidin (PA) composition of 55 plant extracts before and after alkaline oxidation by ultrahigh-resolution UHPLC-MS/MS. We characterized the natural PA structures in detail and studied the sophisticated changes in the modified PA structures and the typical patterns and models of reactions within different PA classes due to the oxidation. The natural PAs were A- and B-type PCs, PDs and PC/PD mixtures. In addition, we detected galloylated PAs. B-type PCs in different plant extracts were rather stable and showed no or minor modification due to the alkaline oxidation. For some samples, we detected the intramolecular reactions of PCs producing A-type ether linkages. A-type PCs were also rather stable with no or minor modification, but in some plants, the formation of additional ether linkages was detected. PAs containing PD units were more reactive. After alkaline oxidation, these PAs or their oxidation products were no longer detected by MS even though a different type and/or delayed PA hump was still detected by UV at 280 nm. Galloylated PAs were rather stable under alkaline oxidation if they were PC-based, but we detected the intramolecular conversion from B-type to A-type. Galloylated PDs were more reactive and reacted similarly to nongalloylated PDs.
Keywords: UHPLC-DAD-MS/MS; high-resolution mass spectrometry; orbitrap; oxidation; tannins.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures








Similar articles
-
Modification of Natural Proanthocyanidin Oligomers and Polymers Via Chemical Oxidation under Alkaline Conditions.ACS Omega. 2021 Feb 9;6(7):4726-4739. doi: 10.1021/acsomega.0c05515. eCollection 2021 Feb 23. ACS Omega. 2021. PMID: 33644580 Free PMC article.
-
Characterization by HPLC-ESI-MS2 of native and oxidized procyanidins from litchi (Litchi chinensis) pericarp.Food Chem. 2019 Sep 1;291:126-131. doi: 10.1016/j.foodchem.2019.04.020. Epub 2019 Apr 5. Food Chem. 2019. PMID: 31006450
-
Alkaline oxidization can increase the in vitro antiparasitic activity of proanthocyanidin-rich plant extracts against Ascarissuum.Exp Parasitol. 2023 May;248:108493. doi: 10.1016/j.exppara.2023.108493. Epub 2023 Mar 6. Exp Parasitol. 2023. PMID: 36889503
-
Two-Dimensional Tannin Fingerprints by Liquid Chromatography Tandem Mass Spectrometry Offer a New Dimension to Plant Tannin Analyses and Help To Visualize the Tannin Diversity in Plants.J Agric Food Chem. 2018 Sep 5;66(35):9162-9171. doi: 10.1021/acs.jafc.8b02115. Epub 2018 Aug 23. J Agric Food Chem. 2018. PMID: 30136834 Free PMC article. Review.
-
MALDI-TOF MS analysis of plant proanthocyanidins.J Pharm Biomed Anal. 2010 Jan 20;51(2):358-72. doi: 10.1016/j.jpba.2009.03.035. Epub 2009 Apr 7. J Pharm Biomed Anal. 2010. PMID: 19410413 Review.
Cited by
-
Exploring the Interactions between Plant Proanthocyanidins and Thiabendazole: Insights from Isothermal Titration Calorimetry.Molecules. 2024 Jul 25;29(15):3492. doi: 10.3390/molecules29153492. Molecules. 2024. PMID: 39124899 Free PMC article.
-
Comparison of the Stability of a Camu Camu Extract Dried and Encapsulated by Means of High-Throughput Electrospraying Assisted by Pressurized Gas.Foods. 2024 Oct 16;13(20):3280. doi: 10.3390/foods13203280. Foods. 2024. PMID: 39456342 Free PMC article.
-
Willow (Salix spp.) bark hot water extracts inhibit both enveloped and non-enveloped viruses: study on its anti-coronavirus and anti-enterovirus activities.Front Microbiol. 2023 Nov 8;14:1249794. doi: 10.3389/fmicb.2023.1249794. eCollection 2023. Front Microbiol. 2023. PMID: 38029113 Free PMC article.
-
Identification of Oxindoleacetic Acid Conjugates in Quinoa (Chenopodium quinoa Willd.) Seeds by High-Resolution UHPLC-MS/MS.Molecules. 2022 Aug 31;27(17):5629. doi: 10.3390/molecules27175629. Molecules. 2022. PMID: 36080392 Free PMC article.
-
Polyphenols and Phenolic Glucosides in Antibacterial Twig Extracts of Naturally Occurring Salix myrsinifolia (Salisb.), S. phylicifolia (L.) and S. starkeana (Willd.) and the Cultivated Hybrid S. x pendulina (Wender.).Pharmaceutics. 2024 Jul 9;16(7):916. doi: 10.3390/pharmaceutics16070916. Pharmaceutics. 2024. PMID: 39065613 Free PMC article.
References
-
- Hemingway R.W. Structural variation in proanthocyanidins and their derivatives. In: Hemingway R.W., Karchesy J.J., editors. Chemistry and Significance of Condensed Tannins. Plenum Press; New York, NY, USA: 1989. pp. 83–107.
-
- Tannin Market Size, Share & Trends Analysis Report by Sources (Plants, Brown Algae), by Product (Hydrolysable, Non-Hydrolysable, Phlorotannins), by Application (Leather Tanning, Wine Production, Wood Adhesives), & Segment Forecasts, 2014–2015. [(accessed on 5 February 2021)]; Available online: https://www.grandviewresearch.com/industry-analysis/tannin-market.
-
- Pizzi A. Tannin-based adhesives. J. Macromol. Sci. Part C. 1980;18:247–325. doi: 10.1080/00222358008081043. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

