Seasonal Variations in the Gut Fungal Communities of Hooded Crane (Grus monacha) at Wintering and Stopover Sites in China
- PMID: 33810386
- PMCID: PMC8067105
- DOI: 10.3390/ani11040941
Seasonal Variations in the Gut Fungal Communities of Hooded Crane (Grus monacha) at Wintering and Stopover Sites in China
Abstract
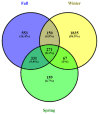
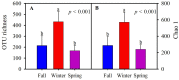
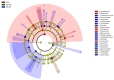
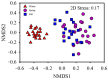
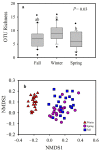
The "gut fungal microbiome" maintains the immune system, homeostasis, and various physiological functions of an organism. Different factors shape and affect gut fungal diversity and community composition, such as environment, habitat type, food resources, and seasons during migration. Wild birds amid migration are exposed to different habitats with different environments, available food resources, and seasons, which may substantially impact their gut fungal community composition and diversity. The hooded crane (Grus monacha) is a known migratory bird that migrates over long distances and is exposed to varied habitats with different environments and food types. We investigated the differences in gut fungal diversity and community composition between wintering and stopover sites amid three migratory seasons. We deduced the gut fungal pathogenic diversity and community composition during winter, fall, and spring by using high throughput sequencing (Illumina Mi-seq), and the internal transcribed region 2 (ITS2) was examined. Samples were collected from Shengjin Lake in the winter and Lindian during the fall and spring. The dominant fungal phyla found across the three seasons were Ascomycota, Basidiomycota, Zygomycota, and Rozellomycota. The gut fungal alpha diversity showed significant shifts during winter at the wintering site compared with the fall and spring seasons at the stopover site. The fungal community composition exhibited a significant change across the three seasons (ANOSIM p = 0.001). The results also demonstrated that the diversity and relative abundance of potential pathogens also showed divergence in winter compared to fall and spring. This study provides the basis for understanding the discrepancy in gut fungal diversity and community composition during migratory seasons at both wintering and stopover grounds. It also suggests that conservation measures should be applied to the conservation of hooded cranes and other wild birds, as the risk of cross-infection increases during seasonal migration.
Keywords: fungi; high-throughput sequencing; hooded crane; migration; pathogens; seasonality.
Conflict of interest statement
No conflict of interest declared by the authors. The funders had no role in the study’s design, in the collection, analyses, or interpretation of the data, in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Chen M., Chen X.Q., Tian L.X., Liu Y.J., Niu J. Enhanced intestinal health, immune responses and ammonia resistance in Pacific white shrimp (Litopenaeus vannamei) fed dietary hydrolyzed yeast (Rhodotorula mucilaginosa) and Bacillus licheniformis. Aquac.Rep. 2020;17:100385. doi: 10.1016/j.aqrep.2020.100385. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

