Androgen Receptor-Dependent Mechanisms Mediating Drug Resistance in Prostate Cancer
- PMID: 33810413
- PMCID: PMC8037957
- DOI: 10.3390/cancers13071534
Androgen Receptor-Dependent Mechanisms Mediating Drug Resistance in Prostate Cancer
Abstract
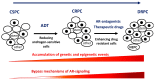
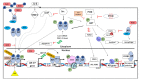
Androgen receptor (AR) is a main driver of prostate cancer (PCa) growth and progression as well as the key drug target. Appropriate PCa treatments differ depending on the stage of cancer at diagnosis. Although androgen deprivation therapy (ADT) of PCa is initially effective, eventually tumors develop resistance to the drug within 2-3 years of treatment onset leading to castration resistant PCa (CRPC). Castration resistance is usually mediated by reactivation of AR signaling. Eventually, PCa develops additional resistance towards treatment with AR antagonists that occur regularly, also mostly due to bypass mechanisms that activate AR signaling. This tumor evolution with selection upon therapy is presumably based on a high degree of tumor heterogenicity and plasticity that allows PCa cells to proliferate and develop adaptive signaling to the treatment and evolve pathways in therapy resistance, including resistance to chemotherapy. The therapy-resistant PCa phenotype is associated with more aggressiveness and increased metastatic ability. By far, drug resistance remains a major cause of PCa treatment failure and lethality. In this review, various acquired and intrinsic mechanisms that are AR‑dependent and contribute to PCa drug resistance will be discussed.
Keywords: AR antagonists; androgen deprivation therapy; androgen receptor; castration resistant PCa; prostate cancer.
Conflict of interest statement
Authors declare no conflict of interest.
Figures
Similar articles
-
Androgen receptors in hormone-dependent and castration-resistant prostate cancer.Pharmacol Ther. 2013 Dec;140(3):223-38. doi: 10.1016/j.pharmthera.2013.07.003. Epub 2013 Jul 13. Pharmacol Ther. 2013. PMID: 23859952 Review.
-
Androgen receptor functions in castration-resistant prostate cancer and mechanisms of resistance to new agents targeting the androgen axis.Oncogene. 2014 May 29;33(22):2815-25. doi: 10.1038/onc.2013.235. Epub 2013 Jun 10. Oncogene. 2014. PMID: 23752196 Free PMC article. Review.
-
MicroRNA Regulation of Androgen Receptor in Castration-Resistant Prostate Cancer: Premises, Promises, and Potentials.Curr Mol Pharmacol. 2021 Oct 25;14(4):559-569. doi: 10.2174/1874467213666201223121850. Curr Mol Pharmacol. 2021. PMID: 33357209 Review.
-
IU1 and enzalutamide combination yields synergistic effects on castration-resistant prostate cancer.Prostate. 2023 Nov;83(15):1446-1457. doi: 10.1002/pros.24607. Epub 2023 Aug 7. Prostate. 2023. PMID: 37545197
-
Adaptive responses of androgen receptor signaling in castration-resistant prostate cancer.Oncotarget. 2015 Nov 3;6(34):35542-55. doi: 10.18632/oncotarget.4689. Oncotarget. 2015. PMID: 26325261 Free PMC article. Review.
Cited by
-
Glucocorticoid Receptor Regulates and Interacts with LEDGF/p75 to Promote Docetaxel Resistance in Prostate Cancer Cells.Cells. 2023 Aug 11;12(16):2046. doi: 10.3390/cells12162046. Cells. 2023. PMID: 37626856 Free PMC article.
-
NEIL3 promotes the carcinogenesis of prostate cancer by activating PI3K/Akt/mTOR signaling.Discov Oncol. 2025 May 30;16(1):967. doi: 10.1007/s12672-025-02625-w. Discov Oncol. 2025. PMID: 40447877 Free PMC article.
-
Strategic Advances in Combination Therapy for Metastatic Castration-Sensitive Prostate Cancer: Current Insights and Future Perspectives.Cancers (Basel). 2024 Sep 18;16(18):3187. doi: 10.3390/cancers16183187. Cancers (Basel). 2024. PMID: 39335158 Free PMC article. Review.
-
New insights into molecular signaling pathways and current advancements in prostate cancer diagnostics & therapeutics.Front Oncol. 2023 Aug 17;13:1193736. doi: 10.3389/fonc.2023.1193736. eCollection 2023. Front Oncol. 2023. Retraction in: Front Oncol. 2023 Dec 07;13:1343833. doi: 10.3389/fonc.2023.1343833. PMID: 37664036 Free PMC article. Retracted. Review.
-
Molecular Similarities and Differences between Canine Prostate Cancer and Human Prostate Cancer Variants.Biomedicines. 2023 Apr 5;11(4):1100. doi: 10.3390/biomedicines11041100. Biomedicines. 2023. PMID: 37189720 Free PMC article. Review.
References
-
- Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. - PubMed
-
- Vander Ark A., Woodford E., Li X. Molecular Mechanisms of Therapies for Prostate Cancer with Bone Metastasis. J. Explor. Res. Pharmacol. 2016;1:35–39.
-
- Crawford E.D., Heidenreich A., Lawrentschuk N., Tombal B., Pompeo A.C., Mendoza-Valdes A., Miller K., Debruyne F.M., Klotz L. Androgen-Targeted Therapy in Men with Prostate Cancer: Evolving Practice and Future Considerations. Prostate Cancer Prostatic Dis. 2019;22:24–38. doi: 10.1038/s41391-018-0079-0. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

