Secondary Metabolites of the Genus Amycolatopsis: Structures, Bioactivities and Biosynthesis
- PMID: 33810439
- PMCID: PMC8037709
- DOI: 10.3390/molecules26071884
Secondary Metabolites of the Genus Amycolatopsis: Structures, Bioactivities and Biosynthesis
Abstract
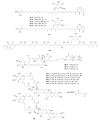
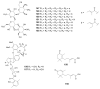
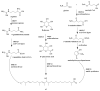
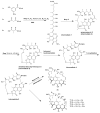
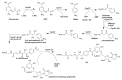
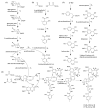
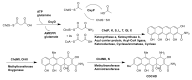
Actinomycetes are regarded as important sources for the generation of various bioactive secondary metabolites with rich chemical and bioactive diversities. Amycolatopsis falls under the rare actinomycete genus with the potential to produce antibiotics. In this review, all literatures were searched in the Web of Science, Google Scholar and PubMed up to March 2021. The keywords used in the search strategy were "Amycolatopsis", "secondary metabolite", "new or novel compound", "bioactivity", "biosynthetic pathway" and "derivatives". The objective in this review is to summarize the chemical structures and biological activities of secondary metabolites from the genus Amycolatopsis. A total of 159 compounds derived from 8 known and 18 unidentified species are summarized in this paper. These secondary metabolites are mainly categorized into polyphenols, linear polyketides, macrolides, macrolactams, thiazolyl peptides, cyclic peptides, glycopeptides, amide and amino derivatives, glycoside derivatives, enediyne derivatives and sesquiterpenes. Meanwhile, they mainly showed unique antimicrobial, anti-cancer, antioxidant, anti-hyperglycemic, and enzyme inhibition activities. In addition, the biosynthetic pathways of several potent bioactive compounds and derivatives are included and the prospect of the chemical substances obtained from Amycolatopsis is also discussed to provide ideas for their implementation in the field of therapeutics and drug discovery.
Keywords: Actinomycetes; Amycolatopsis; antibiotics; biological activities; biosynthetic pathways; chemical structures; natural products.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Secondary Metabolites with Anti-Inflammatory Activities from One Actinobacteria Amycolatopsis taiwanensis.Molecules. 2021 Sep 23;26(19):5765. doi: 10.3390/molecules26195765. Molecules. 2021. PMID: 34641311 Free PMC article.
-
Progress in Understanding the Genetic Information and Biosynthetic Pathways behind Amycolatopsis Antibiotics, with Implications for the Continued Discovery of Novel Drugs.Chembiochem. 2016 Jan;17(2):119-28. doi: 10.1002/cbic.201500542. Epub 2015 Nov 26. Chembiochem. 2016. PMID: 26503579 Review.
-
Amycolachromones A-F, Isolated from a Streptomycin-Resistant Strain of the Deep-Sea Marine Actinomycete Amycolatopsis sp. WP1.Mar Drugs. 2022 Feb 24;20(3):162. doi: 10.3390/md20030162. Mar Drugs. 2022. PMID: 35323461 Free PMC article.
-
The Secondary Metabolites from Genus Kitasatospora: A Promising Source for Drug Discovery.Chem Biodivers. 2024 Dec;21(12):e202401473. doi: 10.1002/cbdv.202401473. Epub 2024 Oct 29. Chem Biodivers. 2024. PMID: 39180497 Review.
-
Chemical Compounds Produced by Bacillus sp. Factories and Their Role in Nature.Mini Rev Med Chem. 2019;19(5):373-380. doi: 10.2174/1389557518666180829113612. Mini Rev Med Chem. 2019. PMID: 30156158 Review.
Cited by
-
Metabolomic analysis in Amycolatopsis keratiniphila disrupted the competing ECO0501 pathway for enhancing the accumulation of vancomycin.World J Microbiol Biotechnol. 2024 Aug 10;40(10):297. doi: 10.1007/s11274-024-04105-9. World J Microbiol Biotechnol. 2024. PMID: 39126539
-
Secondary Metabolites with Anti-Inflammatory Activities from One Actinobacteria Amycolatopsis taiwanensis.Molecules. 2021 Sep 23;26(19):5765. doi: 10.3390/molecules26195765. Molecules. 2021. PMID: 34641311 Free PMC article.
-
Leveraging TME features and multi-omics data with an advanced deep learning framework for improved Cancer survival prediction.Sci Rep. 2025 Apr 24;15(1):14282. doi: 10.1038/s41598-025-98565-0. Sci Rep. 2025. PMID: 40275021 Free PMC article.
-
Bioprospecting for the soil-derived actinobacteria and bioactive secondary metabolites on the Western Qinghai-Tibet Plateau.Front Microbiol. 2023 Oct 11;14:1247001. doi: 10.3389/fmicb.2023.1247001. eCollection 2023. Front Microbiol. 2023. PMID: 37886074 Free PMC article.
-
Exploring the Trends in Actinobacteria as Biological Control Agents of Phytopathogenic Fungi: A (Mini)-Review.Indian J Microbiol. 2024 Mar;64(1):70-81. doi: 10.1007/s12088-023-01166-6. Epub 2024 Jan 12. Indian J Microbiol. 2024. PMID: 38468744 Free PMC article. Review.
References
-
- Lechevalier M.P., Prauser H., Labeda D.P., Ruan J.S. 2 New genera of nocardioform Actinomycetes-Amycolata gen. nov and Amycolatopsis gen. nov. Int. J. Syst. Bacteriol. 1986;36:29–37. doi: 10.1099/00207713-36-1-29. - DOI
-
- Stackebrandt E., Rainey F.A., WardRainey N.L. Proposal for a new hierarchic classification system, actinobacteria classis. Int. J. Syst. Bacteriol. 1997;47:479–491. doi: 10.1099/00207713-47-2-479. - DOI
-
- Sensi P., Greco A.M., Ballotta R. Rifomycin. I. Isolation and properties of rifomycin B and rifomycin complex. Antibiot. Annu. 1959;7:262–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

