PD-(L)1 Inhibitors as Monotherapy for the First-Line Treatment of Non-Small-Cell Lung Cancer Patients with High PD-L1 Expression: A Network Meta-Analysis
- PMID: 33810441
- PMCID: PMC8036854
- DOI: 10.3390/jcm10071365
PD-(L)1 Inhibitors as Monotherapy for the First-Line Treatment of Non-Small-Cell Lung Cancer Patients with High PD-L1 Expression: A Network Meta-Analysis
Abstract
Programmed cell death-ligand 1 (PD-L1) has emerged as a potential biomarker for selection of patients more likely to respond to immunotherapy and as a prognostic factor in non-small cell lung cancer (NSCLC). In this network meta-analysis, we aimed to evaluate the efficacy of first-line anti-PD-(L)1 monotherapy in advanced NSCLC patients with high PD-L1 expression (≥50%) compared to platinum-based chemotherapy. We also evaluated efficacy outcomes according to tumor mutational burden (TMB). To that end, we conducted a systematic review. Six clinical trials with 2111 patients were included. In head-to-head comparisons, immunotherapy showed a significant improvement in progression-free survival (PFS: HRpooled = 0.69, 95% CI: 0.52-0.90, p = 0.007), overall survival (OS: HRpooled = 0.69, 95% CI: 0.61-0.78; p < 0.001) and overall response rate (ORR) (Risk ratio (RR)pooled = 1.354, 95% CI: 1.04-1.762, p = 0.024). In the assessment of relative efficacy for PFS through indirect comparisons, pembrolizumab (results from KEYNOTE-024) ranked highest followed by cemiplimab and atezolizumab, with statistical significance determined for some of the drugs. In terms of OS, cemiplimab ranked highest followed by atezolizumab and pembrolizumab, although non-significant OS was determined for these drugs. In conclusion, PD-(L)1 inhibitor monotherapy improves efficacy outcomes in the first line setting of advanced NSCLC patients with high PD-L1 expression. Evaluations with longer follow up are still needed to determine the superiority of any specific drug.
Keywords: PD-(L)1 inhibitors; efficacy; first-line treatment; immunotherapy; network meta-analysis; non-small cell lung cancer.
Conflict of interest statement
M.M. reports advisory and consultancy honoraria from Roche, Merck, Bristol-Myers Squibb, AstraZeneca, Amgen, Boehringer and Takeda; speaker honoraria from Roche, Merck, Bristol-Myers Squibb, AstraZeneca, Bayer, Amgen and Boehringer; and travel/accommodation expenses from Roche, Merck and Lilly. M.C. reports advisory and consultancy honoraria from Roche, Bristol-Myers Squibb, AstraZeneca and Pfizer; and travel/accommodation expenses from Roche, Bristol-Myers Squibb and AstraZeneca. D.I. reports advisory and consultancy honoraria from Roche, Merck, Bristol-Myers Squibb, AstraZeneca, Lilly, Pfizer, Bayer, Abbvie, Amgen and Boehringer and Takeda; speaker honoraria from Roche, Merck, Bristol-Myers Squibb, AstraZeneca, Lilly, Pfizer, Bayer, Amgen, Boehringer and Sanofi; grant funding from Roche, Bristol-Myers Squibb, AstraZeneca and Lilly; and travel/accommodation expenses from Roche, Merck, Bristol-Myers Squibb, AstraZeneca, Lilly, Pfizer, Amgen and Boehringer. D.M.-M. reports advisory and consultancy honoraria from Roche, Merck, Bristol-Myers Squibb, AstraZeneca, Lilly, Pfizer and Boehringer; and speaker honoraria from Roche, Merck, Bristol-Myers Squibb, AstraZeneca, Lilly, Pfizer and Boehringer. D.R.-A. reports advisory and consultancy honoraria from Roche, Merck, Bristol-Myers Squibb, AstraZeneca, Pfizer, Boehringer and Takeda; speaker honoraria from Roche, Merck, Bristol-Myers Squibb, AstraZeneca, Boehringer and Takeda; and travel/accommodation expenses from Roche, Merck, Bristol-Myers Squibb and AstraZeneca. J.C.-R. reports advisory and consultancy honoraria from Roche and AstraZeneca; and travel/accommodation expenses from Roche. T.M.-B. reports advisory and consultancy honoraria from Roche, Bristol-Myers Squibb, AstraZeneca and Boehringer; speaker honoraria from Merck, Bristol-Myers Squibb and AstraZeneca; and research grants from Kyowa Kirin. R.B.-C. reports advisory honoraria from Roche, Bristol-Myers Squibb and AstraZeneca; and speaker honoraria from Roche, Bristol-Myers Squibb and Amgen. L.P.-A. reports advisory honoraria from Roche, Merck (MSD), Merck Serono, Bristol-Myers Squibb, AstraZeneca, Lilly, Pfizer, PharmaMar, Bayer, Abbvie, Amgen, Janssen, GSK, Novartis, Ipsen, Boehringer, Takeda, Sanofi, Tesaro, Blueprint, Mirati; and research grants from Bristol-Myers Squibb, AstraZeneca and PharmaMar. Additionally, he is a co-founder and board member of Altum Sequencing, as well as an external board member of Genomica. D.P.-P., P.R.-G. and M.M.A. were full-time employees of Roche Farma S.A. at the time the study was conducted.
Figures
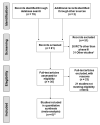

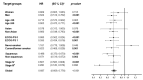
References
-
- Planchard D., Popat S., Kerr K., Novello S., Smit E.F., Faivre-Finn C., Mok T.S., Reck M., Van Schil P.E., Hellmann M.D., et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018;29:iv192–iv237. doi: 10.1093/annonc/mdy275. - DOI - PubMed
-
- Goldstraw P., Chansky K., Crowley J., Rami-Porta R., Asamura H., Eberhardt W.E., Nicholson A.G., Groome P., Mitchell A., Bolejack V. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. - DOI - PubMed
-
- Paz-Ares L.G., de Marinis F., Dediu M., Thomas M., Pujol J.L., Bidoli P., Molinier O., Sahoo T.P., Laack E., Reck M., et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 2013;31:2895–2902. doi: 10.1200/JCO.2012.47.1102. - DOI - PubMed
-
- Liang J., Ming L., Qihai S., Zhengyang H., Yunyi B., Yiwei H., Cheng Z., Wei J., Qun W., Lijie T. Compare the efficacy and safety of programmed cell death-1 (PD-1) and programmed cell death ligand-1 (PD-L1) inhibitors for advanced non-small cell lung cancer: A Bayesian analysis. Transl. Lung Cancer Res. 2020;9:1302–1323. doi: 10.21037/tlcr-20-192. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

