Hepatic Arterial Buffer Response in Liver Radioembolization and Potential Use for Improved Cancer Therapy
- PMID: 33810511
- PMCID: PMC8036746
- DOI: 10.3390/cancers13071537
Hepatic Arterial Buffer Response in Liver Radioembolization and Potential Use for Improved Cancer Therapy
Abstract
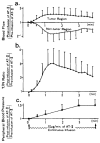
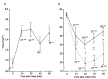
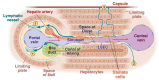
Liver radioembolization is a treatment option for unresectable liver cancers, performed by infusion of 90Y or 166Ho loaded spheres in the hepatic artery. As tumoral cells are mainly perfused via the liver artery unlike hepatic lobules, a twofold tumor to normal liver dose ratio is commonly obtained. To improve tumoral cell killing while preserving lobules, co-infusion of arterial vasoconstrictor has been proposed but with limited success: the hepatic arterial buffer response (HABR) and hepatic vascular escape mechanism hamper the arterioles vasoconstriction. The proposed project aims to take benefit from the HABR by co-infusing a mesenteric arterial vasodilator: the portal flow enhancement inducing the vasoconstriction of the intra sinusoids arterioles barely impacts liver tumors that are mainly fed by novel and anarchic external arterioles. Animal studies were reviewed and dopexamine was identified as a promising safe candidate, reducing by four the hepatic lobules arterial flow. A clinical trial design is proposed. A four to sixfold improvement of the tumoral to normal tissue dose ratio is expected, pushing the therapy towards a real curative intention, especially in HCC where ultra-selective spheres delivery is often not possible.
Keywords: SIRT; TARE; cancer therapy; dose optimization; liver radioembolization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Hepatic arteriolo-portal venular shunting guarantees maintenance of nutritional microvascular supply in hepatic arterial buffer response of rat livers.J Physiol. 2001 Feb 15;531(Pt 1):193-201. doi: 10.1111/j.1469-7793.2001.0193j.x. J Physiol. 2001. PMID: 11179403 Free PMC article.
-
Phase I clinical trial of oral furtulon and combined hepatic arterial chemoembolization and radiotherapy in unresectable primary liver cancers, including clinicopathologic study.Am J Clin Oncol. 2000 Oct;23(5):449-54. doi: 10.1097/00000421-200010000-00004. Am J Clin Oncol. 2000. PMID: 11039502 Clinical Trial.
-
Quantitative comparison of pre-treatment predictive and post-treatment measured dosimetry for selective internal radiation therapy using cone-beam CT for tumor and liver perfusion territory definition.EJNMMI Res. 2020 Aug 14;10(1):94. doi: 10.1186/s13550-020-00675-5. EJNMMI Res. 2020. PMID: 32797332 Free PMC article.
-
Locoregional and systemic therapy for hepatocellular carcinoma.J Gastrointest Oncol. 2017 Apr;8(2):215-228. doi: 10.21037/jgo.2017.03.13. J Gastrointest Oncol. 2017. PMID: 28480062 Free PMC article. Review.
-
Transarterial chemoembolization versus transarterial radioembolization in hepatocellular carcinoma: optimization of selecting treatment modality.Hepatol Int. 2016 Nov;10(6):883-892. doi: 10.1007/s12072-016-9722-9. Epub 2016 Apr 28. Hepatol Int. 2016. PMID: 27126821 Review.
Cited by
-
Optimization of the Clinical Effectiveness of Radioembolization in Hepatocellular Carcinoma with Dosimetry and Patient-Selection Criteria.Curr Oncol. 2022 Mar 29;29(4):2422-2434. doi: 10.3390/curroncol29040196. Curr Oncol. 2022. PMID: 35448170 Free PMC article. Review.
-
Microspheres Used in Liver Radioembolization: From Conception to Clinical Effects.Molecules. 2021 Jun 29;26(13):3966. doi: 10.3390/molecules26133966. Molecules. 2021. PMID: 34209590 Free PMC article. Review.
-
Extreme hepatectomy with modified ALPPS in a rat model: gradual portal vein restriction associated with hepatic artery restriction.BMC Surg. 2023 Sep 25;23(1):291. doi: 10.1186/s12893-023-02197-y. BMC Surg. 2023. PMID: 37749572 Free PMC article.
References
-
- Padia S.A., Lewandowski R.J., Johnson G.E., Sze D.Y., Ward T.J., Gaba R.C., Baerlocher M.O., Gates V.L., Riaz A., Brown D.B., et al. Society of Interventional Radiology Standards of Practice Committee: Radioembolization of hepatic malignancies: Background, quality improvement guidelines, and future directions. J. Vasc. Interv. Radiol. 2017;28:1–5. doi: 10.1016/j.jvir.2016.09.024. - DOI - PubMed
-
- Ilhan H., Goritschan A., Paprottka P., Jakobs T.F., Fendler W.P., Todica A., Bartenstein P., Hacker M., Haug A.R. Predictive value of 99mTc-MAA SPECT for 90Y-labeled resin microsphere distribution in radioembolization of primary and secondary hepatic tumors. J. Nucl. Med. 2015;56:1654–1660. doi: 10.2967/jnumed.115.162685. - DOI - PubMed
-
- Walrand S., Hesse M., Chiesa C., Lhommel R., Jamar F. The low hepatic toxicity per Gray of 90Y glass microspheres is linked to their transport in the arterial tree favoring a nonuniform trapping as observed in posttherapy PET imaging. J. Nucl. Med. 2014;55:135–140. doi: 10.2967/jnumed.113.126839. - DOI - PubMed
-
- Garin E., Tselikas L., Guiu B., Chalaye J., Edeline J., de Baere T., Assenat E., Tacher V., Robert C., Terroir-Cassou-Mounat M., et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): A randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol. Hepatol. 2020;6:17–29. doi: 10.1016/S2468-1253(20)30290-9. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

