Chitosan Membranes Filled with Cyclosporine A as Possible Devices for Local Administration of Drugs in the Treatment of Breast Cancer
- PMID: 33810514
- PMCID: PMC8036521
- DOI: 10.3390/molecules26071889
Chitosan Membranes Filled with Cyclosporine A as Possible Devices for Local Administration of Drugs in the Treatment of Breast Cancer
Abstract
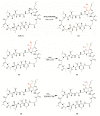
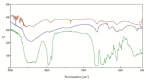
The aim of this work is the design, preparation and characterization of membranes based on cyclosporine A (CsA) and chitosan carboxylate (CC) to be used as an implantable subcutaneous medical device for a prolonged therapeutic effect in the treatment of breast cancer. The choice to use CsA is due to literature data that have demonstrated its possible antitumor activity on different types of neoplastic cells. To this end, CsA was bound to CC through an amidation reaction to obtain a prodrug to be dispersed in a chitosan-based polymeric membrane. The reaction intermediates and the final product were characterized by Fourier transform infrared spectroscopy (FT-IR) and proton nuclear magnetic resonance (1H-NMR). Membranes were analyzed by differential scanning calorimetry (DSC) and scanning electron microscopy (SEM). The data obtained showed the effective formation of the amide bond between CsA and CC and the complete dispersion of CsA inside the polymeric membrane. Furthermore, preliminary tests, conducted on MDA-MB-231, a type of breast cancer cell line, have shown a high reduction in the proliferation of cancer cells. These results indicate the possibility of using the obtained membranes as an interesting strategy for the release of cyclosporin-A in breast cancer patients.
Keywords: breast cancer; carboxylated chitosan; chitosan; cyclosporine A; membrane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Inhibition of the AnxA1/FPR1 autocrine axis reduces MDA-MB-231 breast cancer cell growth and aggressiveness in vitro and in vivo.Biochim Biophys Acta Mol Cell Res. 2018 Sep;1865(9):1368-1382. doi: 10.1016/j.bbamcr.2018.06.010. Epub 2018 Jun 20. Biochim Biophys Acta Mol Cell Res. 2018. PMID: 29932988
-
α-Tocopherol succinate-modified chitosan as a micellar delivery system for paclitaxel: preparation, characterization and in vitro/in vivo evaluations.Int J Pharm. 2012 Feb 28;423(2):480-8. doi: 10.1016/j.ijpharm.2011.12.004. Epub 2011 Dec 13. Int J Pharm. 2012. PMID: 22183133
-
Anti-proliferative effect of chitosan nanoparticles (extracted from crayfish Procambarus clarkii, Crustacea: Cambaridae) against MDA-MB-231 and SK-BR-3 human breast cancer cell lines.Int J Biol Macromol. 2019 Apr 1;126:478-487. doi: 10.1016/j.ijbiomac.2018.12.151. Epub 2018 Dec 17. Int J Biol Macromol. 2019. PMID: 30572045
-
Cyclosporine A inhibits breast cancer cell growth by downregulating the expression of pyruvate kinase subtype M2.Int J Mol Med. 2012 Aug;30(2):302-8. doi: 10.3892/ijmm.2012.989. Epub 2012 May 9. Int J Mol Med. 2012. PMID: 22580449
-
Biodegradable and injectable hydrogels as an immunosuppressive drug delivery system.Mater Sci Eng C Mater Biol Appl. 2019 May;98:472-481. doi: 10.1016/j.msec.2018.11.051. Epub 2019 Jan 4. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 30813049
Cited by
-
Production of α-Tocopherol-Chitosan Nanoparticles by Membrane Emulsification.Molecules. 2022 Apr 3;27(7):2319. doi: 10.3390/molecules27072319. Molecules. 2022. PMID: 35408718 Free PMC article.
-
Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications.Pharmaceutics. 2023 Apr 21;15(4):1313. doi: 10.3390/pharmaceutics15041313. Pharmaceutics. 2023. PMID: 37111795 Free PMC article. Review.
-
Mechanism and Application of Chitosan and Its Derivatives in Promoting Permeation in Transdermal Drug Delivery Systems: A Review.Pharmaceuticals (Basel). 2022 Apr 10;15(4):459. doi: 10.3390/ph15040459. Pharmaceuticals (Basel). 2022. PMID: 35455456 Free PMC article. Review.
-
Preparation and Characterization of Hydrogel Films and Nanoparticles Based on Low-Esterified Pectin for Anticancer Applications.Polymers (Basel). 2023 Aug 2;15(15):3280. doi: 10.3390/polym15153280. Polymers (Basel). 2023. PMID: 37571174 Free PMC article.
-
Non-metabolic enzyme function of pyruvate kinase M2 in breast cancer.Front Oncol. 2024 Oct 1;14:1450325. doi: 10.3389/fonc.2024.1450325. eCollection 2024. Front Oncol. 2024. PMID: 39411137 Free PMC article. Review.
References
-
- Chou H.S., Larsson M., Hsiao M.H., Chen Y.C., Röding M., Nydén M., Liu D.M. Injectable insulin-lysozyme-loaded nanogels with enzymatically-controlled degradation and release for basal insulin treatment: In vitro characterization and in vivo observation. J. Control. Release. 2016;224:33–42. doi: 10.1016/j.jconrel.2015.12.036. - DOI - PubMed
-
- Hsiao M.H., Chiou S.H., Larsson M., Hung K.H., Wang Y.L., Liu C.J., Liu D.M. A temperature-induced and shear-reversible assembly of latanoprost-loaded amphiphilic chitosan colloids: Characterization and in vivo glaucoma treatment. Acta Biomater. 2014;10:3188–3196. doi: 10.1016/j.actbio.2014.03.016. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

