Maintaining Inducibility of Dermal Follicle Cells on Silk Fibroin/Sodium Alginate Scaffold for Enhanced Hair Follicle Regeneration
- PMID: 33810528
- PMCID: PMC8066588
- DOI: 10.3390/biology10040269
Maintaining Inducibility of Dermal Follicle Cells on Silk Fibroin/Sodium Alginate Scaffold for Enhanced Hair Follicle Regeneration
Abstract
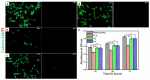
The extracellular matrix (ECM) is important for maintaining cell phenotype and promoting cell proliferation and differentiation. In order to better solve the problem of skin appendage regeneration, a combination of mechanical/enzymatic digestion methods was used to self-extract dermal papilla cells (DPCs), which were seeded on silk fibroin/sodium alginate scaffolds as seed cells to evaluate the possibility of skin regeneration/regeneration of accessory organs. Scanning electron microscopy (SEM) graphs showed that the interconnected pores inside the scaffold had a pore diameter in the range of 153-311 μm and a porosity of 41-82%. Immunofluorescence (IF) staining and cell morphological staining proved that the extracted cells were DPCs. The results of a Cell Counting Kit-8 (CCK-8) and Calcein-AM/PI live-dead cell staining showed that the DPCs grew well in the composite scaffold extract. Normal cell morphology and characteristics of aggregation growth were maintained during the 3-day culture, which showed that the silk fibroin/sodium alginate (SF/SA) composite scaffold had good cell-compatibility. Hematoxylin-eosin (H&E) staining of tissue sections further proved that the cells adhered closely and aggregated to the pore wall of the scaffold, and retained the ability to induce differentiation of hair follicles. All these results indicate that, compared with a pure scaffold, the composite scaffold promotes the adhesion and growth of DPCs. We transplanted the SF/SA scaffolds into the back wounds of SD rats, and evaluated the damage model constructed in vivo. The results showed that the scaffold inoculated with DPCs could accelerate the repair of the skin and promote the regeneration of the hair follicle structure.
Keywords: cell-compatibility; composite scaffold; dermal papilla cells; extracellular matrix; hair follicle structure; self-extract.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Zhang X., Wei X., Liu L., Marti G.P., Ghanamah M.S., Arshad M.J., Strom L., Spence R., Jeng J., Milner S., et al. Association of Increasing Burn Severity in Mice with Delayed Mobilization of Circulating Angiogenic Cells. Arch. Surg. 2010;145:259–266. doi: 10.1001/archsurg.2009.285. - DOI - PMC - PubMed
-
- Da Silva M.A., Crawford A., Mundy J., Correlo V., Sol P., Bhattacharya M., Hatton P., Reis R., Neves N. Chitosan/polyester-based scaffolds for cartilage tissue engineering: Assessment of extracellular matrix formation. Acta Biomater. 2010;6:1149–1157. doi: 10.1016/j.actbio.2009.09.006. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

