Genome Size Covaries More Positively with Propagule Size than Adult Size: New Insights into an Old Problem
- PMID: 33810583
- PMCID: PMC8067107
- DOI: 10.3390/biology10040270
Genome Size Covaries More Positively with Propagule Size than Adult Size: New Insights into an Old Problem
Abstract
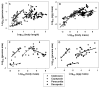
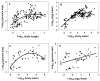
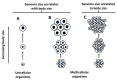
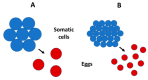
The body size and (or) complexity of organisms is not uniformly related to the amount of genetic material (DNA) contained in each of their cell nuclei ('genome size'). This surprising mismatch between the physical structure of organisms and their underlying genetic information appears to relate to variable accumulation of repetitive DNA sequences, but why this variation has evolved is little understood. Here, I show that genome size correlates more positively with egg size than adult size in crustaceans. I explain this and comparable patterns observed in other kinds of animals and plants as resulting from genome size relating strongly to cell size in most organisms, which should also apply to single-celled eggs and other reproductive propagules with relatively few cells that are pivotal first steps in their lives. However, since body size results from growth in cell size or number or both, it relates to genome size in diverse ways. Relationships between genome size and body size should be especially weak in large organisms whose size relates more to cell multiplication than to cell enlargement, as is generally observed. The ubiquitous single-cell 'bottleneck' of life cycles may affect both genome size and composition, and via both informational (genotypic) and non-informational (nucleotypic) effects, many other properties of multicellular organisms (e.g., rates of growth and metabolism) that have both theoretical and practical significance.
Keywords: Crustacea; allometric scaling; cell size; cellular (nuclear) DNA content; egg and sperm sizes; life cycles; multicellular animals and plants; nucleotypic effects; spore, pollen and seed sizes; unicellular organisms.
Conflict of interest statement
The author declares no conflict of interest.
Figures


References
-
- Peters R.H. The Ecological Implications of Body Size. Cambridge University Press; Cambridge, UK: 1983.
-
- Calder W.A. Size, Function and Life History. Harvard University Press; Cambridge, MA, USA: 1984.
-
- Schmidt-Nielsen K. Scaling: Why Is Animal Size So Important? Cambridge University Press; New York, NY, USA: 1984.
-
- Niklas J.T. Plant. Allometry: The Scaling of Form and Process. University of Chicago Press; Chicago, IL, USA: 1994.
LinkOut - more resources
Full Text Sources
Other Literature Sources

