Diverse Regulation but Conserved Function: SOX9 in Vertebrate Sex Determination
- PMID: 33810596
- PMCID: PMC8066042
- DOI: 10.3390/genes12040486
Diverse Regulation but Conserved Function: SOX9 in Vertebrate Sex Determination
Abstract
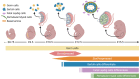
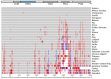
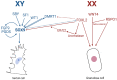
Sex determination occurs early during embryogenesis among vertebrates. It involves the differentiation of the bipotential gonad to ovaries or testes by a fascinating diversity of molecular switches. In most mammals, the switch is SRY (sex determining region Y); in other vertebrates it could be one of a variety of genes including Dmrt1 or dmy. Downstream of the switch gene, SOX9 upregulation is a central event in testes development, controlled by gonad-specific enhancers across the 2 Mb SOX9 locus. SOX9 is a 'hub' gene of gonadal development, regulated positively in males and negatively in females. Despite this diversity, SOX9 protein sequence and function among vertebrates remains highly conserved. This article explores the cellular, morphological, and genetic mechanisms initiated by SOX9 for male gonad differentiation.
Keywords: SOX9; evolution; sex determination; transcription factor; transdifferentiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Stevens N.M. A study of the germ cells of Aphis rosae and Aphis œnotherae. J. Exp. Zool. 1904;2:313–337. doi: 10.1002/jez.1400020302. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

