Anti-Inflammatory Effect of Phytoncide in an Animal Model of Gastrointestinal Inflammation
- PMID: 33810618
- PMCID: PMC8037037
- DOI: 10.3390/molecules26071895
Anti-Inflammatory Effect of Phytoncide in an Animal Model of Gastrointestinal Inflammation
Abstract
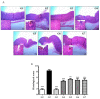
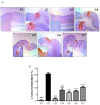
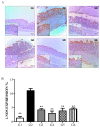
Background: Phytoncide is known to have antimicrobial and anti-inflammatory properties. Purpose: This study was carried out to confirm the anti-inflammatory activity of two types of phytoncide extracts from pinecone waste. Methods: We made two types of animal models to evaluate the efficacy, an indomethacin-induced gastroenteritis rat model and a dextran sulfate sodium-induced colitis mouse model. Result: In the gastroenteritis experiment, the expression of induced-nitric oxide synthase (iNOS), a marker for inflammation, decreased in the phytoncide-supplemented groups, and gastric ulcer development was significantly inhibited (p < 0.05). In the colitis experiment, the shortening of the colon length and the iNOS expression were significantly suppressed in the phytoncide-supplemented group (p < 0.05). Conclusions: Through this study, we confirmed that phytoncide can directly inhibit inflammation in digestive organs. Although further research is needed, we conclude that phytoncide has potential anti-inflammatory properties in the digestive tract and can be developed as a functional agent.
Keywords: Pinus koraiensis; anti-inflammation; colitis; gastritis; phytoncide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Gastroprotective effect of phytoncide extract from Pinus koraiensis pinecone in Helicobacter pylori infection.Sci Rep. 2020 Jun 12;10(1):9547. doi: 10.1038/s41598-020-66603-8. Sci Rep. 2020. PMID: 32533032 Free PMC article.
-
Phytoncide Extracted from Pinecone Decreases LPS-Induced Inflammatory Responses in Bovine Mammary Epithelial Cells.J Microbiol Biotechnol. 2016 Mar;26(3):579-87. doi: 10.4014/jmb.1510.10070. J Microbiol Biotechnol. 2016. PMID: 26608166
-
Aqueous extract of Bruguiera gymnorrhiza leaves protects against dextran sulfate sodium induced ulcerative colitis in mice via suppressing NF-κB activation and modulating intestinal microbiota.J Ethnopharmacol. 2020 Apr 6;251:112554. doi: 10.1016/j.jep.2020.112554. Epub 2020 Jan 7. J Ethnopharmacol. 2020. PMID: 31923541
-
Carvacrol exhibits anti-oxidant and anti-inflammatory effects against 1, 2-dimethyl hydrazine plus dextran sodium sulfate induced inflammation associated carcinogenicity in the colon of Fischer 344 rats.Biochem Biophys Res Commun. 2015 May 29;461(2):314-20. doi: 10.1016/j.bbrc.2015.04.030. Epub 2015 Apr 14. Biochem Biophys Res Commun. 2015. PMID: 25881504
-
Intestinal anti-inflammatory effects of fuzi-ganjiang herb pair against DSS-induced ulcerative colitis in mice.J Ethnopharmacol. 2020 Oct 28;261:112951. doi: 10.1016/j.jep.2020.112951. Epub 2020 Jun 20. J Ethnopharmacol. 2020. PMID: 32574670
Cited by
-
Co-Delivery of Ylang Ylang Oil of Cananga odorata and Oxaliplatin Using Intelligent pH-Sensitive Lipid-Based Nanovesicles for the Effective Treatment of Triple-Negative Breast Cancer.Int J Mol Sci. 2023 May 7;24(9):8392. doi: 10.3390/ijms24098392. Int J Mol Sci. 2023. PMID: 37176099 Free PMC article.
-
Phytochemical-Based Nanoantioxidants Stabilized with Polyvinylpyrrolidone for Enhanced Antibacterial, Antioxidant, and Anti-Inflammatory Activities.Antioxidants (Basel). 2024 Aug 30;13(9):1056. doi: 10.3390/antiox13091056. Antioxidants (Basel). 2024. PMID: 39334715 Free PMC article.
References
-
- Whitfield-Cargile C.M., Cohen N.D., Chapkin R.S., Weeks B.R., Davidson L.A., Goldsby J.S., Hunt C.L., Steinmeyer S.H., Menon R., Suchodolski J.S., et al. The microbiota-derived metabolite indole decreases mucosal inflammation and injury in a murine model of NSAID enteropathy. Gut Microbes. 2016;7:246–261. doi: 10.1080/19490976.2016.1156827. - DOI - PMC - PubMed
-
- Rangarajan S., Rezonzew G., Chumley P., Fatima H., Golovko M.Y., Feng W., Hua P., Jaimes E.A. COX-2-derived prostaglandins as mediators of the deleterious effects of nicotine in chronic kidney disease. Am. J. Physiol. Renal Physiol. 2020;318:F475–F485. doi: 10.1152/ajprenal.00407.2019. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

