The development of an effective synthetic route of rilpivirine
- PMID: 33810807
- PMCID: PMC8017658
- DOI: 10.1186/s13065-021-00749-y
The development of an effective synthetic route of rilpivirine
Abstract
Background: Rilpivirine (RPV) was approved by the U.S. FDA (Food and Drug Administration) in 2011 to treat individuals infected with human immunodeficiency virus 1 (HIV-1). Significantly, rilpivirine is three fold more potent than etravirine. Once-daily, it is used with a low oral dose (25 mg/tablet), decreasing the drug administration and bringing a better choice to the patients. However, there are many shortcomings in the existing synthesis route of RPV, such as the high cost, prolonged reaction time and low yield (18.5%).
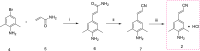
Results: This article describes our efforts to develop an efficient and practical microwave-promoted method to synthesize rilpivirine using less toxic organic reagents and low boiling solvents. The last step's reaction time decreased from 69 h to 90 min through this optimized synthetic procedure, and the overall yield improved from 18.5 to 21%. In addition, the yield of intermediate 3 increased from 52 to 62% compared to the original patent.
Conclusion: Overall, through a series of process optimization, we have developed a practical synthesis method of rilpivirine, which is easy to scale with higher yield and shorter reaction time.
Keywords: Antiviral; HIV; Microwave-promoted method; Rilpivirine; Synthetic optimization.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


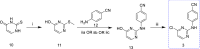
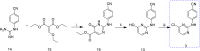
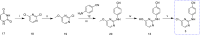


Similar articles
-
Development of a practical synthesis of etravirine via a microwave-promoted amination.Chem Cent J. 2018 Dec 20;12(1):144. doi: 10.1186/s13065-018-0504-4. Chem Cent J. 2018. PMID: 30569261 Free PMC article.
-
Low Frequency of Drug-Resistant Variants Selected by Long-Acting Rilpivirine in Macaques Infected with Simian Immunodeficiency Virus Containing HIV-1 Reverse Transcriptase.Antimicrob Agents Chemother. 2015 Dec;59(12):7762-70. doi: 10.1128/AAC.01937-15. Epub 2015 Oct 5. Antimicrob Agents Chemother. 2015. PMID: 26438501 Free PMC article.
-
Lipid levels and changes in body fat distribution in treatment-naive, HIV-1-Infected adults treated with rilpivirine or Efavirenz for 96 weeks in the ECHO and THRIVE trials.Clin Infect Dis. 2014 Aug 1;59(3):425-34. doi: 10.1093/cid/ciu234. Epub 2014 Apr 11. Clin Infect Dis. 2014. PMID: 24729492 Clinical Trial.
-
Formulation and pharmacology of long-acting rilpivirine.Curr Opin HIV AIDS. 2015 Jul;10(4):233-8. doi: 10.1097/COH.0000000000000164. Curr Opin HIV AIDS. 2015. PMID: 26049947 Review.
-
[Data on rilpivirine in treatment-naïve patients. Lessons from ECHO, THRIVE and STaR].Enferm Infecc Microbiol Clin. 2013 Jun;31 Suppl 2:20-9. doi: 10.1016/S0213-005X(13)70139-3. Enferm Infecc Microbiol Clin. 2013. PMID: 24252530 Review. Spanish.
Cited by
-
The Meandrous Route of Rilpivirine in the Search for the Miraculous Drug to Treat HIV Infections.Viruses. 2025 Jul 8;17(7):959. doi: 10.3390/v17070959. Viruses. 2025. PMID: 40733576 Free PMC article.
-
Non-nucleoside structured compounds with antiviral activity-past 10 years (2010-2020).Eur J Med Chem. 2022 Mar 5;231:114136. doi: 10.1016/j.ejmech.2022.114136. Epub 2022 Jan 19. Eur J Med Chem. 2022. PMID: 35085926 Free PMC article. Review.
References
-
- Kang D, Feng D, Ginex T, Zou J, Wei F, Zhao T, Huang B, Sun Y, Desta S, De Clercq E, et al. Exploring the hydrophobic channel of NNIBP leads to the discovery of novel piperidine-substituted thiophene[3,2-d]pyrimidine derivatives as potent HIV-1 NNRTIs. Acta Pharm Sin B. 2020;10(5):878–894. doi: 10.1016/j.apsb.2019.08.013. - DOI - PMC - PubMed
-
- Wu Y, Tang C, Rui R, Yang L, Ding W, Wang J, Li Y, Lai CC, Wang Y, Luo R, et al. Synthesis and biological evaluation of a series of 2-(((5-akly/aryl-1H-pyrazol-3-yl)methyl)thio)-5-alkyl-6-(cyclohexylmethyl)-pyrimidin-4(3H)-ones as potential HIV-1 inhibitors. Acta Pharm Sin B. 2020;10(3):512–528. doi: 10.1016/j.apsb.2019.08.009. - DOI - PMC - PubMed
Grants and funding
- 81973181/National Natural Science Foundation of China
- 2017CXGC1401/Shandong Provincial Key research and development project
- 2019JZZY021011/Shandong Provincial Key research and development project
- 2019ZX09301126/National Science and Technology Major Projects for "Major New Drugs Innovation and Development"
- GXL20200015001/Foreign cultural and educational experts Project
LinkOut - more resources
Full Text Sources
Other Literature Sources
