Effect of Bicarbonate on Net Acid Excretion, Blood Pressure, and Metabolism in Patients With and Without CKD: The Acid Base Compensation in CKD Study
- PMID: 33810868
- PMCID: PMC9254549
- DOI: 10.1053/j.ajkd.2020.10.015
Effect of Bicarbonate on Net Acid Excretion, Blood Pressure, and Metabolism in Patients With and Without CKD: The Acid Base Compensation in CKD Study
Abstract
Rationale & objective: Patients with CKD are at elevated risk of metabolic acidosis due to impaired net acid excretion (NAE). Identifying early markers of acidosis may guide prevention in chronic kidney disease (CKD). This study compared NAE in participants with and without CKD, as well as the NAE, blood pressure (BP), and metabolomic response to bicarbonate supplementation.
Study design: Randomized order, cross-over study with controlled feeding.
Setting & participants: Participants consisted of 8 patients with CKD (estimated glomerular filtration rate 30-59mL/min/1.73m2 or 60-70mL/min/1.73m2 with albuminuria) and 6 patients without CKD. All participants had baseline serum bicarbonate concentrations between 20 and 28 mEq/L; they did not have diabetes mellitus and did not use alkali supplements at baseline.
Intervention: Participants were fed a fixed-acid-load diet with bicarbonate supplementation (7 days) and with sodium chloride control (7 days) in a randomized order, cross-over fashion.
Outcomes: Urine NAE, 24-hour ambulatory BP, and 24-hour urine and plasma metabolomic profiles were measured after each period.
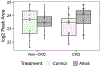
Results: During the control period, mean NAE was 28.3±10.2 mEq/d overall without differences across groups (P=0.5). Urine pH, ammonium, and citrate were significantly lower in CKD than in non-CKD (P<0.05 for each). Bicarbonate supplementation reduced NAE and urine ammonium in the CKD group, increased urine pH in both groups (but more in patients with CKD than in those without), and increased; urine citrate in the CKD group (P< 0.2 for interaction for each). Metabolomic analysis revealed several urine organic anions were increased with bicarbonate in CKD, including 3-indoleacetate, citrate/isocitrate, and glutarate. BP was not significantly changed.
Limitations: Small sample size and short feeding duration.
Conclusions: Compared to patients without CKD, those with CKD had lower acid excretion in the form of ammonium but also lower base excretion such as citrate and other organic anions, a potential compensation to preserve acid-base homeostasis. In CKD, acid excretion decreased further, but base excretion (eg, citrate) increased in response to alkali. Urine citrate should be evaluated as an early and responsive marker of impaired acid-base homeostasis.
Funding: National Institute of Diabetes and Digestive and Kidney Diseases and the Duke O'Brien Center for Kidney Research.
Trial registration: Registered at ClinicalTrials.gov with study number NCT02427594.
Keywords: CKD; NAE; acid-base homeostasis; alkali; bicarbonate; bicarbonate supplementation; chronic kidney disease; cross-over trial; diet; human feeding study; hypertension; metabolic acidosis; net acid excretion; subclinical acidosis.
Copyright © 2021. Published by Elsevier Inc.
Figures




References
-
- Workeneh BT, Mitch WE. Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr. 2010;91(4):1128S–1132S. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

