Ferric carboxymaltose vs. ferrous sulfate for the treatment of anemia in advanced chronic kidney disease: an observational retrospective study and cost analysis
- PMID: 33811227
- PMCID: PMC8018957
- DOI: 10.1038/s41598-021-86769-z
Ferric carboxymaltose vs. ferrous sulfate for the treatment of anemia in advanced chronic kidney disease: an observational retrospective study and cost analysis
Abstract
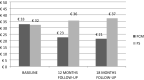
In non-dialysis-dependent chronic kidney disease (NDD-CKD), erythropoiesis-stimulating agents (ESAs) and iron supplementation are essential for anemia management. Ferric carboxymaltose (FCM) is a relatively novel intravenous iron formulation used in different clinical settings, although scarce data exist in NDD-CKD patients. Primary objective of this study was to retrospectively evaluate the efficacy of FCM compared with oral ferrous sulfate for the treatment of iron-deficiency anemia in a cohort of NDD-CKD patients, considering also the treatment costs. This was a monocentric, retrospective observational study reviewing 349 NDD-CKD patients attending an outpatient clinic between June 2013 and December 2016. Patients were treated by either FCM intravenous infusion or oral ferrous sulfate. We collected serum values of hemoglobin, ferritin and transferrin saturation (TSAT) and ESAs doses at 12 and 18 months. The costs related to both treatments were also analysed. 239 patients were treated with FCM intravenous infusion and 110 patients with oral ferrous sulfate. The two groups were not statistically different for age, BMI and eGFR values. At 18 months, hemoglobin, serum ferritin and TSAT values increased significantly from baseline in the FCM group, compared with the ferrous sulfate group. ESAs dose and rate of infusion decreased only in the FCM group. At 18 months, the treatment costs, analysed per week, was higher in the ferrous sulfate group, compared with the FCM group, and this was mostly due to a reduction in ESAs prescription in the FCM group. Routine intravenous FCM treatment in an outpatient clinic of NDD-CKD patients results in better correction of iron-deficiency anemia when compared to ferrous sulfate. In addition to this, treating NDD-CKD patients with FCM leads to a significant reduction of the treatment costs by reducing ESAs use.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Renal function in patients with non-dialysis chronic kidney disease receiving intravenous ferric carboxymaltose: an analysis of the randomized FIND-CKD trial.BMC Nephrol. 2017 Jan 17;18(1):24. doi: 10.1186/s12882-017-0444-6. BMC Nephrol. 2017. PMID: 28095881 Free PMC article. Clinical Trial.
-
Efficacy and safety of ferric carboxymaltose in correcting iron-deficiency anemia: a review of randomized controlled trials across different indications.Arzneimittelforschung. 2010;60(6a):386-98. doi: 10.1055/s-0031-1296303. Arzneimittelforschung. 2010. PMID: 20648930
-
Effect of Ferric Citrate versus Ferrous Sulfate on Iron and Phosphate Parameters in Patients with Iron Deficiency and CKD: A Randomized Trial.Clin J Am Soc Nephrol. 2020 Sep 7;15(9):1251-1258. doi: 10.2215/CJN.15291219. Epub 2020 Jul 21. Clin J Am Soc Nephrol. 2020. PMID: 32694162 Free PMC article. Clinical Trial.
-
Ferric carboxymaltose: a review of its use in iron-deficiency anaemia.Drugs. 2009;69(6):739-56. doi: 10.2165/00003495-200969060-00007. Drugs. 2009. PMID: 19405553 Review.
-
Optimizing iron delivery in the management of anemia: patient considerations and the role of ferric carboxymaltose.Drug Des Devel Ther. 2014 Dec 11;8:2475-91. doi: 10.2147/DDDT.S55499. eCollection 2014. Drug Des Devel Ther. 2014. PMID: 25525337 Free PMC article. Review.
Cited by
-
Ferric carboxymaltose: A game changer in the management of iron deficiency anaemia in pregnancy.J Family Med Prim Care. 2024 Jun;13(6):2379-2384. doi: 10.4103/jfmpc.jfmpc_1258_23. Epub 2024 Jun 14. J Family Med Prim Care. 2024. PMID: 39027861 Free PMC article.
-
Ferric citrate for the treatment of hyperphosphatemia and iron deficiency anaemia in patients with NDD-CKD: a systematic review and meta-analysis.Front Pharmacol. 2024 Mar 7;15:1285012. doi: 10.3389/fphar.2024.1285012. eCollection 2024. Front Pharmacol. 2024. PMID: 38515853 Free PMC article. Review.
-
The Switch from Ferric Gluconate to Ferric Carboxymaltose in Hemodialysis Patients Acts on Iron Metabolism, Erythropoietin, and Costs: A Retrospective Analysis.Medicina (Kaunas). 2023 Jun 2;59(6):1071. doi: 10.3390/medicina59061071. Medicina (Kaunas). 2023. PMID: 37374275 Free PMC article.
-
Effect of Ferric Carboxymaltose Versus Low-Dose Intravenous Iron Therapy and Iron Sucrose on the Total Cost of Care in Patients with Iron Deficiency Anemia: A US Claims Database Analysis.Drugs Real World Outcomes. 2024 Jun;11(2):251-261. doi: 10.1007/s40801-024-00418-1. Epub 2024 Mar 19. Drugs Real World Outcomes. 2024. PMID: 38502304 Free PMC article.
-
Efficacy, Safety and Pharmacoeconomic Analysis of Intravenous Ferric Carboxymaltose in Anemic Hemodialysis Patients Unresponsive to Ferric Gluconate Treatment: A Multicenter Retrospective Study.J Clin Med. 2022 Sep 7;11(18):5284. doi: 10.3390/jcm11185284. J Clin Med. 2022. PMID: 36142929 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous