Platelet and neutrophil responses to Porphyromonas gingivalis in human whole blood
- PMID: 33811483
- PMCID: PMC9281108
- DOI: 10.1111/omi.12336
Platelet and neutrophil responses to Porphyromonas gingivalis in human whole blood
Abstract
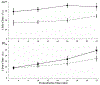
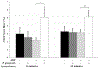
Porphyromonas gingivalis is a causative agent for periodontal disease. Binding of platelets to this gram-negative anaerobe can regulate host hemostatic (thrombus forming) and immune (neutrophil interacting) responses during bacterial infection. Additionally, in response to bacterial pathogens neutrophils can release their DNA, forming highly prothrombotic neutrophil extracellular traps (NETs), which then further enhance platelet responses. This study evaluates the role of P. gingivalis on platelet expression of CD62P, platelet-neutrophil interactions, and labeled neutrophil-associated DNA. Human whole blood was preincubated with varying P. gingivalis concentrations, with or without subsequent addition of adenosine diphosphate (ADP). Flow cytometry was employed to measure platelet expression of CD62P using PerCP-anti-CD61 and PE-anti-CD62P, platelet-neutrophil interactions using PerCP-anti-CD61 and FITC-anti-CD16, and the release of neutrophil DNA using FITC-anti-CD16 and Sytox Blue labeling. Preincubation with a high (6.25 × 106 CFU/mL) level of P. gingivalis significantly increased platelet expression of CD62P in ADP treated and untreated whole blood. In addition, platelet-neutrophil interactions were significantly increased after ADP stimulation, following 5-22 min preincubation of blood with high P. gingivalis CFU. However, in the absence of added ADP, platelet-neutrophil interactions increased in a manner dependent on the preincubation time with P. gingivalis. Moreover, after ADP addition, 16 min preincubation of whole blood with P. gingivalis led to increased labeling of neutrophil-associated DNA. Taken together, the results suggest that the presence of P. gingivalis alters platelet and neutrophil responses to increase platelet activation, platelet interactions with neutrophils, and the level of neutrophil antimicrobial NETs.
Keywords: Porphyromonas gingivalis; flow cytometry; platelet activation; platelet-neutrophil interaction; thrombosis.
© 2021 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
DISCLOSURE OF CONFLICT OF INTERESTS
The authors declare that they have no competing interests.
Figures

Similar articles
-
The periodontal anaerobe Porphyromonas gingivalis induced platelet activation and increased aggregation in whole blood by rat model.Thromb Res. 2011 May;127(5):418-25. doi: 10.1016/j.thromres.2010.12.004. Epub 2011 Feb 18. Thromb Res. 2011. PMID: 21334044
-
Platelet plug formation in whole blood is enhanced in the presence of Porphyromonas gingivalis.Mol Oral Microbiol. 2020 Dec;35(6):251-259. doi: 10.1111/omi.12314. Epub 2020 Oct 10. Mol Oral Microbiol. 2020. PMID: 32949112 Free PMC article.
-
The role of phagocytosis, oxidative burst and neutrophil extracellular traps in the interaction between neutrophils and the periodontal pathogen Porphyromonas gingivalis.Mol Oral Microbiol. 2015 Oct;30(5):361-75. doi: 10.1111/omi.12099. Epub 2015 May 4. Mol Oral Microbiol. 2015. PMID: 25869817
-
Neutrophil Extracellular DNA Traps in Response to Infection or Inflammation, and the Roles of Platelet Interactions.Int J Mol Sci. 2024 Mar 5;25(5):3025. doi: 10.3390/ijms25053025. Int J Mol Sci. 2024. PMID: 38474270 Free PMC article. Review.
-
Interactions Between Neutrophils and Periodontal Pathogens in Late-Onset Periodontitis.Front Cell Infect Microbiol. 2021 Mar 12;11:627328. doi: 10.3389/fcimb.2021.627328. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33777839 Free PMC article. Review.
Cited by
-
Local and Systemic Effects of Porphyromonas gingivalis Infection.Microorganisms. 2023 Feb 13;11(2):470. doi: 10.3390/microorganisms11020470. Microorganisms. 2023. PMID: 36838435 Free PMC article. Review.
-
Apoptotic Vesicles Attenuate Acute Lung Injury via CD73-Mediated Inhibition of Platelet Activation and NETosis.Int J Nanomedicine. 2025 Jan 4;20:91-107. doi: 10.2147/IJN.S485012. eCollection 2025. Int J Nanomedicine. 2025. PMID: 39802376 Free PMC article.
-
Porphyromonas gingivalis regulates atherosclerosis through an immune pathway.Front Immunol. 2023 Mar 14;14:1103592. doi: 10.3389/fimmu.2023.1103592. eCollection 2023. Front Immunol. 2023. PMID: 36999040 Free PMC article. Review.
-
Crosstalk between periodontitis and cardiovascular risk.Front Immunol. 2024 Dec 9;15:1469077. doi: 10.3389/fimmu.2024.1469077. eCollection 2024. Front Immunol. 2024. PMID: 39717783 Free PMC article. Review.
-
Bacterial interactions with platelets: defining key themes.Front Immunol. 2025 Jul 3;16:1610289. doi: 10.3389/fimmu.2025.1610289. eCollection 2025. Front Immunol. 2025. PMID: 40677721 Free PMC article. Review.
References
-
- Blair P, Rex S, Vitseva O, Beaulieu L, Tanriverdi K, Chakrabarti S, Hayashi C, Genco CA, Iafrati M, & Freedman JE (2009). Stimulation of toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circulation Research, 104(3), 346–354. 10.1161/ClRCRESAHA.108.185785 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

