Design and synthesis of small molecular 2-aminobenzoxazoles as potential antifungal agents against phytopathogenic fungi
- PMID: 33811571
- PMCID: PMC8019306
- DOI: 10.1007/s11030-021-10213-7
Design and synthesis of small molecular 2-aminobenzoxazoles as potential antifungal agents against phytopathogenic fungi
Abstract
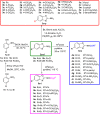
In order to discover novel antifungal agents, three series of simple 2-aminobenzoxazole derivatives were designed, synthesized and evaluated for their antifungal activities against eight phytopathogenic fungi. The in vitro antifungal results showed that most of the target compounds exhibited excellent and broad-spectrum antifungal activities to all the tested fungi. Particularly, the six compounds 3a, 3b, 3c, 3e, 3m and 3v displayed the most potent antifungal activity, with EC50 value of 1.48-16.6 µg/mL, which were much superior to the positive control hymexazol. The in vivo study further confirmed that compounds 3a, 3c, 3e and 3m displayed good preventative effect against Botrytis cinerea at the concentration of 100 µg/mL. The structure-activity relationships research provides significant reference for the further structural optimization of 2-aminobenzoxazole as potential fungicides. Forty-four 2-aminobenzoxazole derivatives were designed and synthesized as agricultural antifungal agents, the in vitro and in vivo antifungal experiments showed that compounds 3a, 3b, 3c, 3e, 3m and 3v exhibited excellent and broad-spectrum antifungal activities compare with the commercial fungicide hymexazol.
Keywords: 2-Aminobenzoxazole derivatives; Antifungal activity; Pathogenic fungi; Structure–activity relationships.
© 2021. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
- 32060627/the National Natural Science Foundation of China (CN)
- [2016]5402/Guizhou Provincial Engineering Laboratory for Chemical Drug R&D
- [2019]1269/Natural Science Foundation of Guizhou Province
- [2020]1Y111/Natural Science Foundation of Guizhou Province
- YJSCXJH[2019]077/The Project of Postgraduate Scientific Research Fund of Guizhou
LinkOut - more resources
Full Text Sources
Other Literature Sources

