The aging lung: Physiology, disease, and immunity
- PMID: 33811810
- PMCID: PMC8052295
- DOI: 10.1016/j.cell.2021.03.005
The aging lung: Physiology, disease, and immunity
Abstract
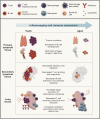
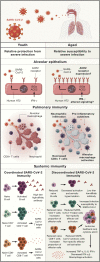
The population is aging at a rate never seen before in human history. As the number of elderly adults grows, it is imperative we expand our understanding of the underpinnings of aging biology. Human lungs are composed of a unique panoply of cell types that face ongoing chemical, mechanical, biological, immunological, and xenobiotic stress over a lifetime. Yet, we do not fully appreciate the mechanistic drivers of lung aging and why age increases the risk of parenchymal lung disease, fatal respiratory infection, and primary lung cancer. Here, we review the molecular and cellular aspects of lung aging, local stress response pathways, and how the aging process predisposes to the pathogenesis of pulmonary disease. We place these insights into context of the COVID-19 pandemic and discuss how innate and adaptive immunity within the lung is altered with age.
Keywords: COPD; COVID-19; aging; healthspan; immunity; inflammaging; lung; lung cancer; metabolism; oxidative stress; proteostasis; pulmonary fibrosis; senescence; stress response.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests C.F.K. is a member of a consortium funded by Bristol-Myers Squibb. A.H.S. is on advisory boards for Surface Oncology, Elstar, SQZ Biotechnologies, Elpiscience, Selecta, Monopteros, and Bicara and consults for Novartis. A.H.S. has received research funding from Novartis, Roche, UCB, Ipsen, Quark, Merck, and AbbVie outside the submitted work. She also is on the scientific advisory boards for the Massachusetts General Cancer Center, Program in Cellular and Molecular Medicine at Boston Children’s Hospital, and the Human Oncology and Pathogenesis Program at Memorial Sloan Kettering Cancer Center and is a scientific editor for the Journal of Experimental Medicine. A.H.S. has patents/pending royalties on the PD-1 pathway from Roche and Novartis. Her spouse has patents/pending royalties on the PD-1/PD-L1 pathway from Roche, Merck MSD, Bristol-Myers-Squibb, Merck KGA, Boehringer-Ingelheim, AstraZeneca, Dako, Leica, Mayo Clinic, and Novartis. He has served on advisory boards for Roche, Bristol-Myers-Squibb, Xios, Origimed, Triursus, iTeos, NextPoint, IgM, Jubilant, Trillium, and GV20 and has equity in Nextpoint, Triursus, Xios, iTeos, IgM, GV20, and Geode. M.C.H. has received research funding from Roche. She is on the advisory board for Pori Therapeutics.
Figures




References
-
- Agrawal A., Agrawal S., Cao J.N., Su H., Osann K., Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J. Immunol. 2007;178:6912–6922. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

