Meibomian gland dysfunction is suppressed via selective inhibition of immune responses by topical LFA-1/ICAM antagonism with lifitegrast in the allergic eye disease (AED) model
- PMID: 33812087
- PMCID: PMC8606044
- DOI: 10.1016/j.jtos.2021.03.009
Meibomian gland dysfunction is suppressed via selective inhibition of immune responses by topical LFA-1/ICAM antagonism with lifitegrast in the allergic eye disease (AED) model
Abstract
Purpose: The etiology of meibomian gland dysfunction (MGD) is incompletely understood, despite being a common ophthalmic condition and an area of unmet medical need. It is characterized by an insufficiency in glandular provision of specialized lipids (meibum) to the tear film and is a major cause of dry eye. Work in the allergic eye disease (AED) mouse model has revealed an immunopathogenic role in MGD causation, now raising interest in the applicability of immunomodulatory therapies. As such, we herein ask whether inhibition of lymphocyte function associated antigen (LFA)-1/intracellular adhesion molecules (ICAM)-1 signaling via topical lifitegrast administration has a therapeutic effect on MGD in AED mice.
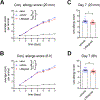
Methods: Mice were induced with AED by i.p. injection of ovalbumin (OVA) mixed with alum and pertussis toxin, followed 2 weeks later by once daily topical OVA challenges for 7 days. Mice were treated topically with 5% lifitegrast ophthalmic solution or vehicle (PBS) 30 min prior to challenge. We developed a clinical ranking method to assess MGD severity, and also scored clinical allergy. Conjunctivae and draining lymph nodes were collected for flow cytometry.
Results: Topical lifitegrast significantly inhibited clinical MGD severity, which was associated with diminished pathogenic TH17 cell and neutrophil numbers in the conjunctiva. No significant change in conjunctival TH2 cells or eosinophils, and only marginal differences in ocular allergy were observed.
Conclusions: In AED mice, lifitegrast inhibited MGD severity marked by a reduction in select immune populations in the conjunctiva. Our findings warrant future examination of lifitegrast in the treatment of patients with forms of MGD.
Keywords: Dry eye; Lifitegrast; MGD; Meibomian gland dysfunction; Neutrophils; T(H)17.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Disclosure/conflicts of interest
Daniel R. Saban has received research funding from Novartis. Victor L. Perez is a consultant for Novartis, Alcon, Kala, EyeGate, Trefoil, and Dompe.
Figures

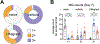


Similar articles
-
A 6-Week, Prospective, Randomized, Single-Masked Study of Lifitegrast Ophthalmic Solution 5% Versus Thermal Pulsation Procedure for Treatment of Inflammatory Meibomian Gland Dysfunction.Cornea. 2020 Apr;39(4):403-407. doi: 10.1097/ICO.0000000000002235. Cornea. 2020. PMID: 31895884 Clinical Trial.
-
Clinical Outcomes of Lifitegrast 5% Ophthalmic Solution in the Treatment of Dry Eye Disease.Eye Contact Lens. 2020 Jan;46 Suppl 1:S20-S24. doi: 10.1097/ICL.0000000000000601. Eye Contact Lens. 2020. PMID: 30985492
-
Aggregated neutrophil extracellular traps occlude Meibomian glands during ocular surface inflammation.Ocul Surf. 2021 Apr;20:1-12. doi: 10.1016/j.jtos.2020.12.005. Epub 2021 Jan 2. Ocul Surf. 2021. PMID: 33401018
-
LipiFlow for the treatment of dry eye disease.Cochrane Database Syst Rev. 2024 Feb 5;2(2):CD015448. doi: 10.1002/14651858.CD015448.pub2. Cochrane Database Syst Rev. 2024. PMID: 38314898 Free PMC article.
-
Lifitegrast Ophthalmic Solution 5%: A Review in Dry Eye Disease.Drugs. 2017 Feb;77(2):201-208. doi: 10.1007/s40265-016-0681-1. Drugs. 2017. PMID: 28058622 Review.
Cited by
-
Ocular surface immune cell diversity in dry eye disease.Indian J Ophthalmol. 2023 Apr;71(4):1237-1247. doi: 10.4103/IJO.IJO_2986_22. Indian J Ophthalmol. 2023. PMID: 37026254 Free PMC article. Review.
-
Meibomian Gland Dysfunction: A Route of Ocular Graft-Versus-Host Disease Progression That Drives a Vicious Cycle of Ocular Surface Inflammatory Damage.Am J Ophthalmol. 2023 Mar;247:42-60. doi: 10.1016/j.ajo.2022.09.009. Epub 2022 Sep 23. Am J Ophthalmol. 2023. PMID: 36162534 Free PMC article.
-
Role of Chinese Medicine Monomers in Dry Eye Disease: Breaking the Vicious Cycle of Inflammation.Pharmacol Res Perspect. 2025 Apr;13(2):e70077. doi: 10.1002/prp2.70077. Pharmacol Res Perspect. 2025. PMID: 39979080 Free PMC article. Review.
-
Comparison of Sodium-Glucose Cotransporter 2 Inhibitors vs Glucagonlike Peptide-1 Receptor Agonists and Incidence of Dry Eye Disease in Patients With Type 2 Diabetes in Taiwan.JAMA Netw Open. 2022 Sep 1;5(9):e2232584. doi: 10.1001/jamanetworkopen.2022.32584. JAMA Netw Open. 2022. PMID: 36136333 Free PMC article.
-
The Yin and Yang of non-immune and immune responses in meibomian gland dysfunction.Ocul Surf. 2024 Apr;32:81-90. doi: 10.1016/j.jtos.2024.01.004. Epub 2024 Jan 14. Ocul Surf. 2024. PMID: 38224775 Free PMC article.
References
-
- Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for. MGD. Investigative ophthalmology & visual science 2011;52:1994–2005. 10.1167/iovs.10-6997e. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

