Evaluation of the Allplex™ GI-Helminth(I) Assay, the first marketed multiplex PCR for helminth diagnosis
- PMID: 33812465
- PMCID: PMC8019563
- DOI: 10.1051/parasite/2021034
Evaluation of the Allplex™ GI-Helminth(I) Assay, the first marketed multiplex PCR for helminth diagnosis
Abstract
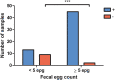
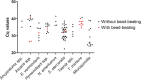
Molecular biology has been gaining more importance in parasitology. Recently, a commercial multiplex PCR assay detecting helminths was marketed: the Allplex™ GI-Helminth(I) Assay. It targets Ancylostoma spp., Ascaris spp., Enterobius vermicularis, Hymenolepis spp., Necator americanus, Strongyloides spp., Taenia spp. and Trichuris trichiura, but also the two most common microsporidia genera in human health, i.e. Enterocytozoon spp. and Encephalitozoon spp. This study aimed to evaluate and compare the Allplex™ GI-Helminth(I) Assay to classical diagnostic methods, based on a cohort of 110 stool samples positive for helminths (microscopy) or for microsporidia (PCR). Samples were stored at -80 °C until analysis by the Allplex™ GI-Helminth(I) Assay. False-negatives were re-tested with bead-beating pretreatment. Without mechanical lysis, concordance and agreement between microscopy and Allplex™ GI-Helminth(I) Assay ranged from 91% to 100% and from 0.15 to 1.00, respectively depending on the target. Concordance was perfect for Taenia spp. (n = 5) and microsporidia (n = 10). False-negative results were observed in 54% (6/13), 34% (4/11) and 20% (7/35) of cases, for hookworms, E. vermicularis and Strongyloides spp. detection, respectively. For these targets, pretreatment improved the results, but only slightly. Trichuris trichiura detection was critically low without pretreatment, as only 9% (1/11) of the samples were positive, but detection reached 91% (10/11) with bead-beating pretreatment. Mechanical lysis was also needed for Ascaris spp. and Hymenolepis spp. to reduce false-negative results from 1/8 to 1/21, respectively, to none for both. Overall, with an optimized extraction process, the Allplex™ GI-Helminth(I) Assay allows the detection of numerous parasites with roughly equivalent performance to that of microscopy, except for hookworms.
Title: Évaluation du kit Allplex™ GI-Helminth(I) Assay, la première PCR multiplex commercialisée pour le diagnostic des helminthes.
Abstract: La biologie moléculaire a maintenant une place importante en parasitologie. Le kit Allplex™ GI-Helminth(I) Assay est le premier panel multiplex commercialisé détectant des helminthes : Ancylostoma spp., Ascaris spp., Enterobius vermicularis, Hymenolepis spp., Necator americanus, Strongyloides spp., Taenia spp. et Trichuris trichiura, mais également les deux genres de Microsporidies les plus fréquents en santé humaine, Enterocytozoon spp. et Encephalitozoon spp. Cette étude a comparé la PCR Allplex™ GI-Helminth(I) Assay aux techniques diagnostiques usuelles, sur une banque préservée à −80 °C, comprenant 110 échantillons de selles positifs à helminthes (microscopie) ou à microsporidies (PCR). Les faux négatifs ont été retestés après prétraitement par broyage en billes. Sans lyse mécanique, la concordance et l’accord entre la microscopie et le test Allplex™ GI-Helminth(I) Assay variaient respectivement de 91 % à 100 % et de 0,15 à 1,00, selon la cible. La concordance était parfaite pour Taenia spp. (n = 5) et les microsporidies (n = 10). Des faux négatifs ont été observés pour la détection des ankylostomes, E. vermicularis et Strongyloides spp. à des taux respectifs de 54 % (6/13), 34 % (4/11) et 20 % (7/35). Pour ces cibles, le prétraitement a peu amélioré les résultats. La détection de T. trichiura était défectueuse sans prétraitement, avec 9 % (1/11) de positifs, mais a atteint 91 % (10/11) après prétraitement par broyage en billes. La lyse mécanique était également nécessaire pour Ascaris spp. et Hymenolepis spp. pour réduire les faux négatifs de 1/8 et 1/21, respectivement, à aucun pour les deux. Au total, avec une optimisation de l’étape d’extraction, le test Allplex™ GI-Helminth(I) Assay permet la détection de nombreux parasites avec des performances proches de celles de la microscopie, excepté pour les ankylostomes.
Keywords: Ascaris spp.; Enterobius vermicularis; Hookworms; Strongyloides spp.; Taenia spp..
© B. Autier et al., published by EDP Sciences, 2021.
Figures
References
-
- Basuni M, Muhi J, Othman N, Verweij JJ, Ahmad M, Miswan N, Rahumatullah A, Aziz FA, Zainudin NS, Noordin R. 2011. A pentaplex real-time polymerase chain reaction assay for detection of four species of soil-transmitted helminths. American Journal of Tropical Medicine and Hygiene, 84, 338–343. - PMC - PubMed
-
- Becker SL, Piraisoody N, Kramme S, Marti H, Silué KD, Panning M, Nickel B, Kern WV, Herrmann M, Hatz CF, N’Goran EK, Utzinger J, von Müller L. 2015. Real-time PCR for detection of Strongyloides stercoralis in human stool samples from Côte d’Ivoire: diagnostic accuracy, inter-laboratory comparison and patterns of hookworm co-infection. Acta Tropica, 150, 210–217. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
