Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors
- PMID: 33812495
- PMCID: PMC8016402
- DOI: 10.1016/S1470-2045(21)00155-8
Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors
Erratum in
-
Correction to Lancet Oncol 2021; 22: 581-83.Lancet Oncol. 2021 Jun;22(6):e239. doi: 10.1016/S1470-2045(21)00264-3. Epub 2021 Apr 30. Lancet Oncol. 2021. PMID: 33939964 Free PMC article. No abstract available.
Conflict of interest statement
IW and BW contributed to conception and design of the study. BW, AA, ES, and HP contributed to collection and analysis of data. BW and IW contributed to data analysis and interpretation. All authors contributed to writing of the Comment and approved the final version before submission. This study had no funding. BW reports speaker fees and fees for consultancy from MSD. AA reports speaker fees and fees for consultancy from MSD, Roche, Boehringer Ingelheim, AstraZeneca, Eli Lilly, Pfizer, Bristol Meyers Squibb, Novartis, Merck Serono, Sanofi, Oncotest-Teva, Medison, AbbVie, and Takeda; grants from Altman Health; and is a member of the board for the Israeli Lung Cancer Advocacy Group. IW reports speaker fees and fees for consultancy from Roche, Bristol Myers Squibb, MSD, and Novartis, and research funding from Roche and MSD. ES and HP declare no competing interests. No individual participant-level data will be shared.
Figures
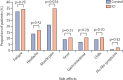
References
-
- Harel A. Israel's ambitious vaccine drive is set for success as Netanyahu seeks election campaign boost. Haaretz. Dec 29, 2020. https://www.haaretz.com/israel-news/.premium-israel-s-ambitious-vaccine-...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

