Impact of differences in acute respiratory distress syndrome randomised controlled trial inclusion and exclusion criteria: systematic review and meta-analysis
- PMID: 33812666
- PMCID: PMC9768208
- DOI: 10.1016/j.bja.2021.02.027
Impact of differences in acute respiratory distress syndrome randomised controlled trial inclusion and exclusion criteria: systematic review and meta-analysis
Abstract
Background: Control-arm mortality varies between acute respiratory distress syndrome (ARDS) RCTs.
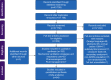
Methods: We systematically reviewed ARDS RCTs that commenced recruitment after publication of the American-European Consensus (AECC) definition (MEDLINE, Embase, and Cochrane central register of controlled trials; January 1994 to October 2020). We assessed concordance of RCT inclusion criteria to ARDS consensus definitions and whether exclusion criteria are strongly or poorly justified. We estimated the proportion of between-trial difference in control-arm 28-day mortality explained by the inclusion criteria and RCT design characteristics using meta-regression.
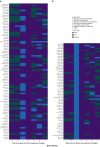
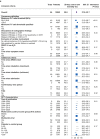
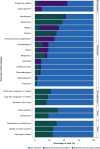
Results: A literature search identified 43 709 records. One hundred and fifty ARDS RCTs were included; 146/150 (97.3%) RCTs defined ARDS inclusion criteria using AECC/Berlin definitions. Deviations from consensus definitions, primarily aimed at improving ARDS diagnostic certainty, frequently related to duration of hypoxaemia (117/146; 80.1%). Exclusion criteria could be grouped by rationale for selection into strongly or poorly justified criteria. Common poorly justified exclusions included pregnancy related, age, and comorbidities (infectious/immunosuppression, hepatic, renal, and human immunodeficiency virus/acquired immunodeficiency syndrome). Control-arm 28-day mortality varied between ARDS RCTs (mean: 29.8% [95% confidence interval: 27.0-32.7%; I2=88.8%; τ2=0.02; P<0.01]), and differed significantly between RCTs with different Pao2:FiO2 ratio inclusion thresholds (26.6-39.9 kPa vs <26.6 kPa; P<0.01). In a meta-regression model, inclusion criteria and RCT design characteristics accounted for 30.6% of between-trial difference (P<0.01).
Conclusions: In most ARDS RCTs, consensus definitions are modified to use as inclusion criteria. Between-RCT mortality differences are mostly explained by the Pao2:FiO2 ratio threshold within the consensus definitions. An exclusion criteria framework can be applied when designing and reporting exclusion criteria in future ARDS RCTs.
Keywords: ARDS; exclusion; inclusion; mortality; randomised controlled trial.
Copyright © 2021 British Journal of Anaesthesia. Published by Elsevier Ltd. All rights reserved.
Figures

References
-
- Bernard G.R., Artigas A., Brigham K.L., et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. - PubMed
-
- Force A.D.T., Ranieri V.M., Rubenfeld G.D., et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. - PubMed
-
- Phua J., Badia J.R., Adhikari N.K.J., et al. Has mortality from acute respiratory distress syndrome decreased over time? Am J Respir Crit Care Med. 2009;179:220–227. - PubMed
-
- Villar J., Perez-Mendez L., Belda J., et al. An early PEEP/FIO2 trial identifies different degrees of lung injury in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007;176:795–804. - PubMed
-
- Villar J., Perez-Mendez L., Blanco J., et al. A universal definition of ARDS: the PaO2/FiO2 ratio under a standard ventilatory setting—a prospective, multicenter validation study. Intensive Care Med. 2013;39:583–592. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

