Mini-review: Microtubule sliding in neurons
- PMID: 33812935
- PMCID: PMC8097519
- DOI: 10.1016/j.neulet.2021.135867
Mini-review: Microtubule sliding in neurons
Abstract
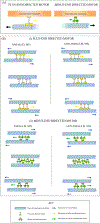
Microtubule sliding is an underappreciated mechanism that contributes to the establishment, organization, preservation, and plasticity of neuronal microtubule arrays. Powered by molecular motor proteins and regulated in part by static crosslinker proteins, microtubule sliding is the movement of microtubules relative to other microtubules or to non-microtubule structures such as the actin cytoskeleton. In addition to other important functions, microtubule sliding significantly contributes to the establishment and maintenance of microtubule polarity patterns in different regions of the neuron. The purpose of this article is to review the state of knowledge on microtubule sliding in the neuron, with emphasis on its mechanistic underpinnings as well as its functional significance.
Keywords: Axon; Cytoplasmic dynein; KIFC1; Kinesin-1; Microtubule; Microtubule crosslinking; Microtubule polarity orientation; Microtubule polarity sorting; Microtubule sliding; Microtubule transport; TRIM46.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures




Similar articles
-
Regulation of Axonal Microtubule Polarity Orientation in Different Kinds of Neurons.FASEB J. 2025 Jun 15;39(11):e70683. doi: 10.1096/fj.202500675RR. FASEB J. 2025. PMID: 40458980
-
Polarity Sorting of Microtubules in the Axon.Trends Neurosci. 2018 Feb;41(2):77-88. doi: 10.1016/j.tins.2017.11.002. Epub 2017 Nov 30. Trends Neurosci. 2018. PMID: 29198454 Free PMC article. Review.
-
Cytoplasmic Dynein Transports Axonal Microtubules in a Polarity-Sorting Manner.Cell Rep. 2017 Jun 13;19(11):2210-2219. doi: 10.1016/j.celrep.2017.05.064. Cell Rep. 2017. PMID: 28614709 Free PMC article.
-
Interplay between kinesin-1 and cortical dynein during axonal outgrowth and microtubule organization in Drosophila neurons.Elife. 2015 Dec 28;4:e10140. doi: 10.7554/eLife.10140. Elife. 2015. PMID: 26615019 Free PMC article.
-
Differentiation between Oppositely Oriented Microtubules Controls Polarized Neuronal Transport.Neuron. 2017 Dec 20;96(6):1264-1271.e5. doi: 10.1016/j.neuron.2017.11.018. Epub 2017 Nov 30. Neuron. 2017. PMID: 29198755 Free PMC article. Review.
Cited by
-
Regulation of Axonal Microtubule Polarity Orientation in Different Kinds of Neurons.FASEB J. 2025 Jun 15;39(11):e70683. doi: 10.1096/fj.202500675RR. FASEB J. 2025. PMID: 40458980
-
Nuclear movement in multinucleated cells.Development. 2022 Nov 1;149(21):dev200749. doi: 10.1242/dev.200749. Epub 2022 Oct 28. Development. 2022. PMID: 36305464 Free PMC article. Review.
-
Principles of microtubule polarity in linear cells.Dev Biol. 2022 Mar;483:112-117. doi: 10.1016/j.ydbio.2022.01.004. Epub 2022 Jan 8. Dev Biol. 2022. PMID: 35016908 Free PMC article. Review.
-
How neurons maintain their axons long-term: an integrated view of axon biology and pathology.Front Neurosci. 2023 Jul 26;17:1236815. doi: 10.3389/fnins.2023.1236815. eCollection 2023. Front Neurosci. 2023. PMID: 37564364 Free PMC article. Review.
-
Centrosome-dependent microtubule modifications set the conditions for axon formation.Cell Rep. 2022 Apr 19;39(3):110686. doi: 10.1016/j.celrep.2022.110686. Cell Rep. 2022. PMID: 35443171 Free PMC article.
References
-
- Lasek RJ, Polymer sliding in axons, Journal of cell science. Supplement, 5 (1986) 161–179. - PubMed
-
- Baas PW, Buster DW, Slow axonal transport and the genesis of neuronal morphology, Journal of neurobiology, 58 (2004) 3–17. - PubMed
-
- Okabe S, Hirokawa N, Turnover of fluorescently labelled tubulin and actin in the axon, Nature, 343 (1990) 479–482. - PubMed
-
- Takeda S, Funakoshi T, Hirokawa N, Tubulin dynamics in neuronal axons of living zebrafish embryos, Neuron, 14 (1995) 1257–1264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

