Molecular properties of human guanylate cyclase-activating protein 2 (GCAP2) and its retinal dystrophy-associated variant G157R
- PMID: 33812995
- PMCID: PMC8113879
- DOI: 10.1016/j.jbc.2021.100619
Molecular properties of human guanylate cyclase-activating protein 2 (GCAP2) and its retinal dystrophy-associated variant G157R
Abstract
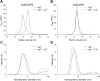
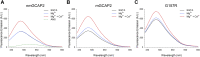
In murine and bovine photoreceptors, guanylate cyclase-activating protein 2 (GCAP2) activates retinal guanylate cyclases (GCs) at low Ca2+ levels, thus contributing to the Ca2+/cGMP negative feedback on the cyclase together with its paralog guanylate cyclase-activating protein 1, which has the same function but different Ca2+ sensitivity. In humans, a GCAP2 missense mutation (G157R) has been associated with inherited retinal degeneration (IRD) via an unknown molecular mechanism. Here, we characterized the biochemical properties of human GCAP2 and the G157R variant, focusing on its dimerization and the Ca2+/Mg2+-binding processes in the presence or absence of N-terminal myristoylation. We found that human GCAP2 and its bovine/murine orthologs significantly differ in terms of oligomeric properties, cation binding, and GC regulation. Myristoylated GCAP2 endothermically binds up to 3 Mg2+ with high affinity and forms a compact dimer that may reversibly dissociate in the presence of Ca2+. Conversely, nonmyristoylated GCAP2 does not bind Mg2+ over the physiological range and remains as a monomer in the absence of Ca2+. Both myristoylated and nonmyristoylated GCAP2 bind Ca2+ with high affinity. At odds with guanylate cyclase-activating protein 1 and independently of myristoylation, human GCAP2 does not significantly activate retinal GC1 in a Ca2+-dependent fashion. The IRD-associated G157R variant is characterized by a partly misfolded, molten globule-like conformation with reduced affinity for cations and prone to form aggregates, likely mediated by hydrophobic interactions. Our findings suggest that GCAP2 might be mostly implicated in processes other than phototransduction in human photoreceptors and suggest a possible molecular mechanism for G157R-associated IRD.
Keywords: GCAP; GUCA1B; cGMP; calcium-binding proteins; guanylate cyclase (guanylyl cyclase); neurodegenerative disease; phototransduction; retina; retinal degeneration; vision.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures







References
-
- Palczewski K., Polans A.S., Baehr W., Ames J.B. Ca(2+)-binding proteins in the retina: Structure, function, and the etiology of human visual diseases. Bioessays. 2000;22:337–350. - PubMed
-
- Koch K.W., Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature. 1988;334:64–66. - PubMed
-
- Hwang J.Y., Koch K.W. Calcium- and myristoyl-dependent properties of guanylate cyclase-activating protein-1 and protein-2. Biochemistry. 2002;41:13021–13028. - PubMed
-
- Peshenko I.V., Moiseyev G.P., Olshevskaya E.V., Dizhoor A.M. Factors that determine Ca2+ sensitivity of photoreceptor guanylyl cyclase. Kinetic analysis of the interaction between the Ca2+-bound and the Ca2+-free guanylyl cyclase activating proteins (GCAPs) and recombinant photoreceptor guanylyl cyclase 1 (RetGC-1) Biochemistry. 2004;43:13796–13804. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

