Recommendations for accurate genotyping of SARS-CoV-2 using amplicon-based sequencing of clinical samples
- PMID: 33813118
- PMCID: PMC8016543
- DOI: 10.1016/j.cmi.2021.03.029
Recommendations for accurate genotyping of SARS-CoV-2 using amplicon-based sequencing of clinical samples
Abstract
Objectives: Genotyping of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been instrumental in monitoring viral evolution and transmission during the pandemic. The quality of the sequence data obtained from these genotyping efforts depends on several factors, including the quantity/integrity of the input material, the technology, and laboratory-specific implementation. The current lack of guidelines for SARS-CoV-2 genotyping leads to inclusion of error-containing genome sequences in genomic epidemiology studies. We aimed to establish clear and broadly applicable recommendations for reliable virus genotyping.
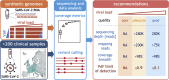
Methods: We established and used a sequencing data analysis workflow that reliably identifies and removes technical artefacts; such artefacts can result in miscalls when using alternative pipelines to process clinical samples and synthetic viral genomes with an amplicon-based genotyping approach. We evaluated the impact of experimental factors, including viral load and sequencing depth, on correct sequence determination.
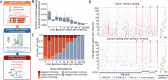
Results: We found that at least 1000 viral genomes are necessary to confidently detect variants in the SARS-CoV-2 genome at frequencies of ≥10%. The broad applicability of our recommendations was validated in over 200 clinical samples from six independent laboratories. The genotypes we determined for clinical isolates with sufficient quality cluster by sampling location and period. Our analysis also supports the rise in frequencies of 20A.EU1 and 20A.EU2, two recently reported European strains whose dissemination was facilitated by travel during the summer of 2020.
Conclusions: We present much-needed recommendations for the reliable determination of SARS-CoV-2 genome sequences and demonstrate their broad applicability in a large cohort of clinical samples.
Keywords: Amplicon; Coronavirus; Genome; Genotyping; Guidelines; NGS; Next-generation sequencing; Recommendations; SARS-CoV-2.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

