The applicability of Magseed® for targeted axillary dissection in breast cancer patients treated with neoadjuvant chemotherapy
- PMID: 33813230
- PMCID: PMC8050798
- DOI: 10.1016/j.breast.2021.03.008
The applicability of Magseed® for targeted axillary dissection in breast cancer patients treated with neoadjuvant chemotherapy
Abstract
Background: Targeted axillary dissection (TAD), the combination of sentinel lymph node biopsy (SLNB) and targeted lymph node biopsy (TLNB), can reduce the false negative rates of sentinel node biopsy alone dramatically in breast cancer patients, who received neoadjuvant chemotherapy (NAC). However methods for TAD are still under investigation.
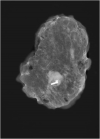
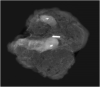
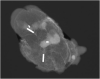
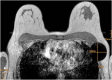
Methods: Magseed®, a non-radioactive magnetic marker was used to mark the biopsied positive TLN after NAC. The SLNB with the standard technetium-based method and the selective TLNB with Magseed® localization were performed in 40 patients. The TLNs were identified with the Sentimag® probe and excised in all patients. Specimen x-ray was performed to confirm the Magseed® within the prior to NAC biopsied and clipped lymph node.
Results: The TLN identification rate was 100% (40/40), the SLN identification rate was 82.5% (33/40), the concordance rate between the TLN and the SLN was 65% (26/40). Complications according Magseed® deployment or identification could not be observed.
Conclusion: Magseed® is a reliable and feasible marker for the identification of TLNs after NAC.
Keywords: Magnetic marker; Targeted axillary dissection; Targeted lymph node biopsy.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest None of the authors has to disclose any commercial interest in the subject of the study, and there was no financial or material support.
Figures
References
-
- Kuehn T., Bauerfeind I., Fehm T. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609–618. - PubMed
-
- Boileau J.F., Poirier B., Basik M. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33(3):258–264. - PubMed
-
- Caudle A.S., Yang W.T., Krishnamurthy S., Mittendorf E.A., Black D.M. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34(10):1072–1078. - PMC - PubMed
-
- Donker M., Straver M.E., Wesseling J. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg. 2015;261(2):378–382. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous