Single individual structural variant detection uncovers widespread hemizygosity in molluscs
- PMID: 33813894
- PMCID: PMC8059565
- DOI: 10.1098/rstb.2020.0153
Single individual structural variant detection uncovers widespread hemizygosity in molluscs
Abstract
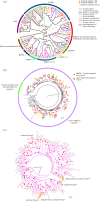
The advent of complete genomic sequencing has opened a window into genomic phenomena obscured by fragmented assemblies. A good example of these is the existence of hemizygous regions of autosomal chromosomes, which can result in marked differences in gene content between individuals within species. While these hemizygous regions, and presence/absence variation of genes that can result, are well known in plants, firm evidence has only recently emerged for their existence in metazoans. Here, we use recently published, complete genomes from wild-caught molluscs to investigate the prevalence of hemizygosity across a well-known and ecologically important clade. We show that hemizygous regions are widespread in mollusc genomes, not clustered in individual chromosomes, and often contain genes linked to transposition, DNA repair and stress response. With targeted investigations of HSP70-12 and C1qDC, we also show how individual gene families are distributed within pan-genomes. This work suggests that extensive pan-genomes are widespread across the conchiferan Mollusca, and represent useful tools for genomic evolution, allowing the maintenance of additional genetic diversity within the population. As genomic sequencing and re-sequencing becomes more routine, the prevalence of hemizygosity, and its impact on selection and adaptation, are key targets for research across the tree of life. This article is part of the Theo Murphy meeting issue 'Molluscan genomics: broad insights and future directions for a neglected phylum'.
Keywords: genome; hemizygosity; mollusc; pan-genome; presence/absence variation; structural variation.
Figures



References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
