Lipidomics Prediction of Parkinson's Disease Severity: A Machine-Learning Analysis
- PMID: 33814463
- PMCID: PMC8355022
- DOI: 10.3233/JPD-202476
Lipidomics Prediction of Parkinson's Disease Severity: A Machine-Learning Analysis
Abstract
Background: The role of the lipidome as a biomarker for Parkinson's disease (PD) is a relatively new field that currently only focuses on PD diagnosis.
Objective: To identify a relevant lipidome signature for PD severity markers.
Methods: Disease severity of 149 PD patients was assessed by the Unified Parkinson's Disease Rating Scale (UPDRS) and the Montreal Cognitive Assessment (MoCA). The lipid composition of whole blood samples was analyzed, consisting of 517 lipid species from 37 classes; these included all major classes of glycerophospholipids, sphingolipids, glycerolipids, and sterols. To handle the high number of lipids, the selection of lipid species and classes was consolidated via analysis of interrelations between lipidomics and disease severity prediction using the random forest machine-learning algorithm aided by conventional statistical methods.
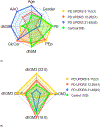
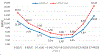
Results: Specific lipid classes dihydrosphingomyelin (dhSM), plasmalogen phosphatidylethanolamine (PEp), glucosylceramide (GlcCer), dihydro globotriaosylceramide (dhGB3), and to a lesser degree dihydro GM3 ganglioside (dhGM3), as well as species dhSM(20:0), PEp(38:6), PEp(42:7), GlcCer(16:0), GlcCer(24:1), dhGM3(22:0), dhGM3(16:0), and dhGB3(16:0) contribute to PD severity prediction of UPDRS III score. These, together with age, age at onset, and disease duration, also contribute to prediction of UPDRS total score. We demonstrate that certain lipid classes and species interrelate differently with the degree of severity of motor symptoms between men and women, and that predicting intermediate disease stages is more accurate than predicting less or more severe stages.
Conclusion: Using machine-learning algorithms and methodologies, we identified lipid signatures that enable prediction of motor severity in PD. Future studies should focus on identifying the biological mechanisms linking GlcCer, dhGB3, dhSM, and PEp with PD severity.
Keywords: PD severity; Parkinson’s disease (PD); UPDRS; lipidome; lipidomics; machine learning.
Conflict of interest statement
Conflict of interest
The authors have no conflict of interest to report.
Figures





References
-
- Di Pasquale E, Fantini J, Chahinian H, Maresca M, Taieb N, Yahi N (2010) Altered ion channel formation by the Parkinson’s-disease-linked E46K mutant of alpha-synuclein is corrected by GM3 but not by GM1 gangliosides. J Mol Biol 397, 202–218. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

