Pathophysiology of Neurogenic Obesity After Spinal Cord Injury
- PMID: 33814879
- PMCID: PMC7983633
- DOI: 10.46292/sci20-00067
Pathophysiology of Neurogenic Obesity After Spinal Cord Injury
Abstract
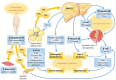
Individuals with a spinal cord injury (SCI) have a unique physiology characterized by sarcopenia, neurogenic osteoporosis, neurogenic anabolic deficiency, sympathetic dysfunction, and blunted satiety associated with their SCI, all of which alter energy balance and subsequently body composition. The distinct properties of "neurogenic obesity" place this population at great risk for metabolic dysfunction, including systemic inflammation, hyperglycemia, dyslipidemia, and hypertension. The purpose of this article is to demonstrate the relationship between neurogenic obesity and the metabolic syndrome after SCI, highlighting the mechanisms associated with adipose tissue pathology and those respective comorbidities. Additionally, representative studies of persons with SCI will be provided to elucidate the severity of the problem and to prompt greater vigilance among SCI specialists as well as primary care providers in order to better manage the epidemic from a public health perspective.
Keywords: dyslipidemia; glucose intolerance; hypertension; metabolic syndrome; obesity; spinal cord injury.
© 2021 American Spinal Injury Association.
Figures
Similar articles
-
Interrelationship of Neurogenic Obesity and Chronic Neuropathic Pain in Persons With Spinal Cord Injury.Top Spinal Cord Inj Rehabil. 2021;27(1):75-83. doi: 10.46292/sci20-00062. Top Spinal Cord Inj Rehabil. 2021. PMID: 33814885 Free PMC article. Review.
-
Relationship Between Sleep-Disordered Breathing and Neurogenic Obesity in Adults With Spinal Cord Injury.Top Spinal Cord Inj Rehabil. 2021;27(1):84-91. doi: 10.46292/sci20-00044. Top Spinal Cord Inj Rehabil. 2021. PMID: 33814886 Free PMC article. Review.
-
Energy Expenditure Following Spinal Cord Injury: A Delicate Balance.Top Spinal Cord Inj Rehabil. 2021;27(1):92-99. doi: 10.46292/sci20-00030. Top Spinal Cord Inj Rehabil. 2021. PMID: 33814887 Free PMC article. Review.
-
The Physiology of Neurogenic Obesity: Lessons from Spinal Cord Injury Research.Obes Facts. 2023;16(4):313-325. doi: 10.1159/000530888. Epub 2023 May 22. Obes Facts. 2023. PMID: 37231872 Free PMC article. Review.
-
Neurogenic obesity and systemic inflammation following spinal cord injury: A review.J Spinal Cord Med. 2018 Jul;41(4):378-387. doi: 10.1080/10790268.2017.1357104. Epub 2017 Jul 30. J Spinal Cord Med. 2018. PMID: 28758554 Free PMC article. Review.
Cited by
-
Diagnosis and Management of Cardiovascular Risk in Individuals With Spinal Cord Injury: A Narrative Review.Circulation. 2023 Jul 18;148(3):268-277. doi: 10.1161/CIRCULATIONAHA.123.064859. Epub 2023 Jul 17. Circulation. 2023. PMID: 37459417 Free PMC article. Review.
-
Preliminary observations on the administration of a glucagon-like peptide-1 receptor agonist on body weight and select carbohydrate endpoints in persons with spinal cord injury: A controlled case series.J Spinal Cord Med. 2024 Jul;47(4):597-604. doi: 10.1080/10790268.2023.2207064. Epub 2023 May 9. J Spinal Cord Med. 2024. PMID: 37158751 Free PMC article. Clinical Trial.
-
The use of alkaline phosphatase as a bone turnover marker after spinal cord injury: A scoping review of human and animal studies.J Spinal Cord Med. 2023 Mar;46(2):167-180. doi: 10.1080/10790268.2021.1977905. Epub 2021 Dec 22. J Spinal Cord Med. 2023. PMID: 34935593 Free PMC article.
-
Treatment of obesity in spinal cord injury with tirzepatide: a case report.Spinal Cord Ser Cases. 2025 Mar 1;11(1):4. doi: 10.1038/s41394-025-00699-w. Spinal Cord Ser Cases. 2025. PMID: 40025019 Free PMC article.
-
Interaction between increasing body mass index and spinal cord injury to the probability of developing a diagnosis of nonalcoholic fatty liver disease.Obes Sci Pract. 2022 Nov 1;9(3):253-260. doi: 10.1002/osp4.643. eCollection 2023 Jun. Obes Sci Pract. 2022. PMID: 37287523 Free PMC article.
References
-
- Bray GA, Kim KK, Wilding JPH. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18(7):715–723. - PubMed
-
- World Health Organization Obesity: preventing and managing the global epidemic. WHO Tech Rep Ser. 2000;(894):i–xii. 1–253. - PubMed
-
- Pigeyre M, Yazdi Fereshteh T, Kaur Y et al. Recent progress in genetics, epigenetics and metagenomics unveils the pathophysiology of human obesity. Clin Sci. 2016;130(12):943–986. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
