Effectiveness and Safety of Iguratimod in Treating Primary Sjögren's Syndrome: A Systematic Review and Meta-Analysis
- PMID: 33815105
- PMCID: PMC8017188
- DOI: 10.3389/fphar.2021.621208
Effectiveness and Safety of Iguratimod in Treating Primary Sjögren's Syndrome: A Systematic Review and Meta-Analysis
Abstract
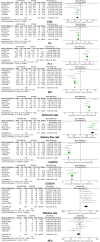
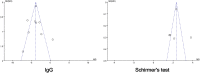
Objectives: We aimed to assess the effectiveness and safety of iguratimod (IGU) in treating primary Sjögren's syndrome (pSS) by meta-analysis. Methods: Eight databases and two clinical trial websites were searched from conception to August 10, 2020, for relevant randomized controlled trials (RCTs) on outcomes of patients with pSS treated with IGU. Revman 5.4 was used for statistical analysis and creating plots. Results: A total of 1,384 patients with pSS from 19 RCTs were included in this meta-analysis. Pooled results demonstrated that patients treated with IGU + hydroxychloroquine (HCQ) + glucocorticoid (GC) showed significant differences in erythrocyte sedimentation rate (ESR), rheumatoid factor (RF) level, platelet (PLT) count, immunoglobulin G (IgG) level, salivary flow rate, Schirmer's test result, EULAR Sjögren's Syndrome Patient Reported Index (ESSPRI), EULAR Sjögren's Syndrome Disease Activity Index (ESSDAI), and efficacy rate (p ≤ 0.01) compared to patients treated with HCQ + GC. Compared to treatment with HCQ and GC, co-administration of IGU with GC showed significant differences in ESR and RF level (p ≤ 0.01); however, no significant differences were noted in IgG level. Conversely, the IgG level showed a significant improvement in the IGU + HCQ + GC group compared to the HCQ + GC group. The results of safety analysis revealed that seven trials showed no significant differences in adverse events (AEs) between the IGU + HCQ + GC and HCQ + GC groups (p = 0.15). Although no severe AEs were noted, gastrointestinal discomfort was the most common AE in the IGU group. No significant differences in AEs were observed between the IGU + GC and HCQ + GC groups. Conclusion: IGU improved the clinical symptoms of patients with pSS, including inflammatory indicators (ESR, IgG, and RF levels), PLT count, secretion function of the salivary and lacrimal glands (salivary flow rate and Schirmer's test result), and disease indexes (ESSDAI and ESSPRI), when co-administered with HCQ + GC therapy without increasing the risks of AEs. Therefore, IGU can be considered as an effective and safe drug for clinical therapy of pSS. Considering the limitations of the present trials, more long-term, multicenter, and high-quality RCTs are required to assess the effectiveness and safety of IGU for treating patients with pSS.
Keywords: effectiveness; iguratimod; meta-analysis; primary Sjögren’s syndrome; safety.
Copyright © 2021 Pu, Wang, Riaz, Zhang, Gao, Pan, Wu, Liang, Zhuang and Tang.
Conflict of interest statement
The authors declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Efficacy and safety of iguratimod combined with methylprednisolone for primary Sjögren's syndrome: a meta-analysis and trial sequential analysis.Eur Rev Med Pharmacol Sci. 2023 Aug;27(16):7544-7556. doi: 10.26355/eurrev_202308_33406. Eur Rev Med Pharmacol Sci. 2023. PMID: 37667931
-
Comparison of the effect of iguratimod and hydroxychloroquine on regulatory B cells in the treatment of primary Sjögren's syndrome.Front Med (Lausanne). 2025 Jul 3;12:1573973. doi: 10.3389/fmed.2025.1573973. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40678132 Free PMC article.
-
The Effectiveness and Safety of Total Glucosides of Paeony in Primary Sjögren's Syndrome: A Systematic Review and Meta-Analysis.Front Pharmacol. 2019 May 24;10:550. doi: 10.3389/fphar.2019.00550. eCollection 2019. Front Pharmacol. 2019. PMID: 31178729 Free PMC article.
-
Efficacy and safety of iguratimod in the treatment of rheumatic and autoimmune diseases: a meta-analysis and systematic review of 84 randomized controlled trials.Front Pharmacol. 2023 Dec 7;14:1189142. doi: 10.3389/fphar.2023.1189142. eCollection 2023. Front Pharmacol. 2023. PMID: 38143490 Free PMC article.
-
Efficacy and safety of iguratimod on patients with primary Sjögren's syndrome: a randomized, placebo-controlled clinical trial.Scand J Rheumatol. 2021 Mar;50(2):143-152. doi: 10.1080/03009742.2020.1809701. Epub 2020 Oct 29. Scand J Rheumatol. 2021. PMID: 33118847 Clinical Trial.
Cited by
-
Research progress on the clinical application and mechanism of iguratimod in the treatment of autoimmune diseases and rheumatic diseases.Front Immunol. 2023 Sep 21;14:1150661. doi: 10.3389/fimmu.2023.1150661. eCollection 2023. Front Immunol. 2023. PMID: 37809072 Free PMC article. Review.
-
Carboxyamidotriazole Regulates the Function of Salivary Gland Epithelial Cells and B Cells to Alleviate Experimental Sjögren's Disease in Mice.Int J Med Sci. 2025 Apr 28;22(10):2362-2372. doi: 10.7150/ijms.111897. eCollection 2025. Int J Med Sci. 2025. PMID: 40386058 Free PMC article.
-
Management of Sjogren's Dry Eye Disease-Advances in Ocular Drug Delivery Offering a New Hope.Pharmaceutics. 2022 Dec 31;15(1):147. doi: 10.3390/pharmaceutics15010147. Pharmaceutics. 2022. PMID: 36678777 Free PMC article. Review.
-
A Systematic Review and Meta-Analysis of 19 Randomized Controlled Trials of Iguratimod Combined With Other Therapies for Sjogren's Syndrome.Front Immunol. 2022 Jul 28;13:924730. doi: 10.3389/fimmu.2022.924730. eCollection 2022. Front Immunol. 2022. PMID: 35967307 Free PMC article.
-
Iguratimod in treatment of primary Sjögren's syndrome concomitant with autoimmune hemolytic anemia: A case report.World J Clin Cases. 2022 Feb 6;10(4):1286-1290. doi: 10.12998/wjcc.v10.i4.1286. World J Clin Cases. 2022. PMID: 35211561 Free PMC article.
References
-
- Bai J., Jiao Y. (2019). Observation on the clinical effect of iguratimod in treatment of primary Sjogren's syndrome. Shanxi Med. J. 48 (14), 1724–1726. 10.3969/j.issn.0253-9926.2019.14.034 - DOI
-
- Bodewes I. L. A., Gottenberg J. E., van Helden-Meeuwsen C. G., Mariette X., Versnel M. A. (2020). Hydroxychloroquine treatment downregulates systemic interferon activation in primary Sjögren's syndrome in the JOQUER randomized trial. Rheuma. (Oxford) 59 (1), 107–111. 10.1093/rheumatology/kez242 - DOI - PMC - PubMed
-
- Chinese Rheumatology Association (2010). Guidelines for diagnosis and treatment of Sjögren’s syndrome. Chin. J. Rheumatol. 14, 766–768. 10.3760/cma.j.issn.1007-7480.2010.11.011 - DOI
-
- Fasano S., Isenberg D. A. (2019). Present and novel biologic drugs in primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 37 (Suppl. 118), 167–174. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

