Treatment Switching and Discontinuation Over 20 Years in the Big Multiple Sclerosis Data Network
- PMID: 33815259
- PMCID: PMC8010264
- DOI: 10.3389/fneur.2021.647811
Treatment Switching and Discontinuation Over 20 Years in the Big Multiple Sclerosis Data Network
Abstract
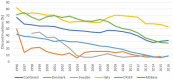
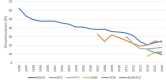
Background: Although over a dozen disease modifying treatments (DMTs) are available for relapsing forms of multiple sclerosis (MS), treatment interruption, switching and discontinuation are common challenges. The objective of this study was to describe treatment interruption and discontinuation in the Big MS data network. Methods: We merged information on 269,822 treatment episodes in 110,326 patients from 1997 to 2016 from five clinical registries in this cohort study. Treatment stop was defined as a clinician recorded DMT end for any reason and included treatment interruptions, switching to alternate DMTs and long-term or permanent discontinuations. Results: The incidence of DMT stopping cross the full observation period was lowest in FTY (19.7 per 100 person-years (PY) of treatment; 95% CI 19.2-20.1), followed by NAT (22.6/100 PY; 95% CI 22.2-23.0), IFNβ (23.3/100 PY; 95% CI 23.2-23.5). Of the 184,013 observed DMT stops, 159,309 (86.6%) switched to an alternate DMT within 6 months. Reasons for stopping a drug were stable during the observation period with lack of efficacy being the most common reason followed by lack of tolerance and side effects. The proportion of patients continuing on most DMTs were similarly stable until 2014 and 2015 when drop from 83 to 75% was noted. Conclusions: DMT stopping reasons and rates were mostly stable over time with a slight increase in recent years, with the availability of more DMTs. The overall results suggest that discontinuation of MS DMTs is mostly due to DMT properties and to a lesser extent to risk management and a competitive market.
Keywords: big MS data; disease modifying treatment; multiple sclerosis; registry study; treatment interruption and discontinuation.
Copyright © 2021 Hillert, Magyari, Soelberg Sørensen, Butzkueven, Van Der Welt, Vukusic, Trojano, Iaffaldano, Pellegrini, Hyde, Stawiarz, Manouchehrinia and Spelman.
Conflict of interest statement
TS received compensation for serving on scientific advisory boards, honoraria for consultancy and funding for travel from Biogen; speaker honoraria from Novartis. MM has served on scientific advisory board for Biogen Idec and Teva and has received honoraria for lecturing from Biogen Idec, Merck Serono, Sanofi-Aventis and Teva. MM has received support for congress participation from Biogen Idec, Merck Serono, Novartis and Genzyme. PS has served on scientific advisory boards for Merck Serono, Teva, Novartis, Sanofi-Aventis and Biogen Idec and has received research support from Biogen Idec, Novartis and Sanofi-Aventis and received speaker honoraria from Merck Serono, Novartis, Teva, Sanofi-Aventis, Biogen Idec and Genzyme. HB received compensation for serving on scientific advisory boards and as a consultant for Biogen, Novartis; speaker honoraria from Biogen Australia, Merck Serono Australia, Novartis Australia; travel support from Biogen Australia, Merck Serono Australia; research support from the CASS Foundation (Australia), Merck Serono Australia, the Royal Melbourne Hospital. SV received consulting and lecturing fees, travel grants and research support from Biogen, Celgene, Genentech, Genzyme, Medday pharmaceuticals, Merck Serono, Novartis, Roche, Sanofi Aventis, and Teva Pharma. MT has served on scientific Advisory Boards for Biogen, Novartis, Roche, and Genzyme; has received speaker honoraria and travel support from Biogen Idec, Sanofi-Aventis, Merck Serono, Teva, Genzyme and Novartis; and has received research grants for her Institution from Biogen Idec, Merck Serono, and Novartis. PI has served on scientific advisory boards for Biogen Idec, Bayer, Teva, Roche, Merck Serono, Novartis, and Genzyme and has received funding for travel and/or Speaker honoraria from Sanofi Aventis, Genzyme, Biogen Idec, Teva, Merck Serono, and Novartis. FP is an employee of Biogen. RH is an employee of Biogen and holds stock. JH has received honoraria for serving on advisory boards for Biogen, Sanofi-Genzyme, and Novartis and speaker's fees from Biogen, Novartis, Merck-Serono, Bayer-Schering, Teva, and Sanofi-Genzyme. JH has served as PI for projects, or received unrestricted research support from BiogenIdec, Merck-Serono, TEVA, Sanofi-Genzyme, and Bayer-Schering. JH MS research is funded by the Swedish Research Council and the Swedish Brain Foundation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Costello K, Kennedy P, Scanzillo J. Recognizing nonadherence in patients with multiple sclerosis and maintaining treatment adherence in the long term. Medscape J Med. (2008) 10:225. Available online at: https://pubmed.ncbi.nlm.nih.gov/19008986/ - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

