The Antitumor Peptide ERα17p Exerts Anti-Hyperalgesic and Anti-Inflammatory Actions Through GPER in Mice
- PMID: 33815268
- PMCID: PMC8011567
- DOI: 10.3389/fendo.2021.578250
The Antitumor Peptide ERα17p Exerts Anti-Hyperalgesic and Anti-Inflammatory Actions Through GPER in Mice
Abstract
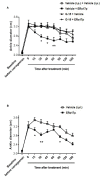
Persistent inflammation and persistent pain are major medical, social and economic burdens. As such, related pharmacotherapy needs to be continuously improved. The peptide ERα17p, which originates from a part of the hinge region/AF2 domain of the human estrogen receptor α (ERα), exerts anti-proliferative effects in breast cancer cells through a mechanism involving the hepta-transmembrane G protein-coupled estrogen receptor (GPER). It is able to decrease the size of xenografted human breast tumors, in mice. As GPER has been reported to participate in pain and inflammation, we were interested in exploring the potential of ERα17p in this respect. We observed that the peptide promoted anti-hyperalgesic effects from 2.5 mg/kg in a chronic mice model of paw inflammation induced by the pro-inflammatory complete Freund's adjuvant (CFA). This action was abrogated by the specific GPER antagonist G-15, leading to the conclusion that a GPER-dependent mechanism was involved. A systemic administration of a Cy5-labeled version of the peptide allowed its detection in both, the spinal cord and brain. However, ERα17p-induced anti-hyperalgesia was detected at the supraspinal level, exclusively. In the second part of the study, we have assessed the anti-inflammatory action of ERα17p in mice using a carrageenan-evoked hind-paw inflammation model. A systemic administration of ERα17p at a dose of 2.5 mg/kg was responsible for reduced paw swelling. Overall, our work strongly suggests that GPER inverse agonists, including ERα17p, could be used to control hyperalgesia and inflammation.
Keywords: ERα17p; GPER; hyperalgesia; inflammation; pain.
Copyright © 2021 Mallet, Boudieu, Lamoine, Coudert, Jacquot and Eschalier.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
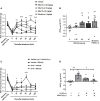
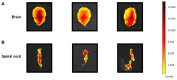
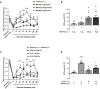
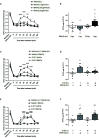
References
-
- Liu N-J, Chakrabarti S, Schnell S, Wessendorf M, Gintzler AR. Spinal synthesis of estrogen and concomitant signaling by membrane estrogen receptors regulate spinal κ- and μ-opioid receptor heterodimerization and female-specific spinal morphine antinociception. J Neurosci (2011) 31:11836–45. 10.1523/JNEUROSCI.1901-11.2011 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

