Astrocyte Clocks and Glucose Homeostasis
- PMID: 33815298
- PMCID: PMC8015704
- DOI: 10.3389/fendo.2021.662017
Astrocyte Clocks and Glucose Homeostasis
Abstract
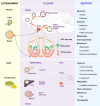
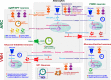
The endogenous timekeeping system evolved to anticipate the time of the day through the 24 hours cycle of the Earth's rotation. In mammals, the circadian clock governs rhythmic physiological and behavioral processes, including the daily oscillation in glucose metabolism, food intake, energy expenditure, and whole-body insulin sensitivity. The results from a series of studies have demonstrated that environmental or genetic alterations of the circadian cycle in humans and rodents are strongly associated with metabolic diseases such as obesity and type 2 diabetes. Emerging evidence suggests that astrocyte clocks have a crucial role in regulating molecular, physiological, and behavioral circadian rhythms such as glucose metabolism and insulin sensitivity. Given the concurrent high prevalence of type 2 diabetes and circadian disruption, understanding the mechanisms underlying glucose homeostasis regulation by the circadian clock and its dysregulation may improve glycemic control. In this review, we summarize the current knowledge on the tight interconnection between the timekeeping system, glucose homeostasis, and insulin sensitivity. We focus specifically on the involvement of astrocyte clocks, at the organism, cellular, and molecular levels, in the regulation of glucose metabolism.
Keywords: astrocytes; circadian clock; diabetes; glucose homeostasis; metabolism.
Copyright © 2021 Barca-Mayo and López.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

