Mumps Orchitis: Clinical Aspects and Mechanisms
- PMID: 33815357
- PMCID: PMC8013702
- DOI: 10.3389/fimmu.2021.582946
Mumps Orchitis: Clinical Aspects and Mechanisms
Abstract
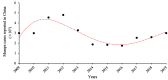
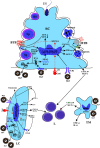
The causative agent of mumps is a single-stranded, non-segmented, negative sense RNA virus belonging to the Paramyxoviridae family. Besides the classic symptom of painfully swollen parotid salivary glands (parotitis) in mumps virus (MuV)-infected men, orchitis is the most common form of extra-salivary gland inflammation. Mumps orchitis frequently occurs in young adult men, and leads to pain and swelling of the testis. The administration of MuV vaccines in children has been proven highly effective in reducing the incidence of mumps. However, a recent global outbreak of mumps and the high rate of orchitis have recently been considered as threats to male fertility. The pathogenesis of mumps orchitis remains largely unclear due to lack of systematic clinical data analysis and animal models studies. The alarming increase in the incidence of mumps orchitis and the high risk of the male fertility have thus become a major health concern. Recent studies have revealed the mechanisms by which MuV-host cells interact and MuV infection induces inflammatory responses in testicular cells. In this mini-review, we highlight advances in our knowledge of the clinical aspects and possible mechanisms of mumps orchitis.
Keywords: MuV; infertility; mumps; orchitis; testis.
Copyright © 2021 Wu, Wang, Tang and Han.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Ternavasio-de la Vega HG, Boronat M, Ojeda A, Garcia-Delgado Y, Angel-Moreno A, Carranza-Rodriguez C, et al. . Mumps orchitis in the post-vaccine era (1967-2009): a single-center series of 67 patients and review of clinical outcome and trends. Med (Baltimore) (2010) 89:96–116. 10.1097/MD.0b013e3181d63191 - DOI - PubMed
-
- Rozina EE, Hilgenfeldt M. Comparative study on the neurovirulence of different vaccine strains of parotitis virus in monkeys. Acta Virol (1985) 29:225–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

