Anti-Inflammatory (M2) Response Is Induced by a sp2-Iminosugar Glycolipid Sulfoxide in Diabetic Retinopathy
- PMID: 33815384
- PMCID: PMC8013727
- DOI: 10.3389/fimmu.2021.632132
Anti-Inflammatory (M2) Response Is Induced by a sp2-Iminosugar Glycolipid Sulfoxide in Diabetic Retinopathy
Abstract
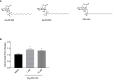
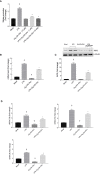
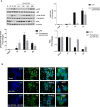
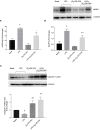
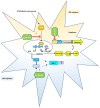
Diabetic retinopathy (DR) is one of the most common complications of Diabetes Mellitus (DM) and is directly associated with inflammatory processes. Currently, neuro-inflammation is considered an early event in DR and proceeds via microglia polarization. A hallmark of DR is the presence of retinal reactive gliosis. Here we report the beneficial effect of (SS,1R)-1-docecylsulfiny-5N,6O-oxomethylidenenojirimycin ((Ss)-DS-ONJ), a member of the sp2-iminosugar glycolipid (sp2-IGL) family, by decreasing iNOS and inflammasome activation in Bv.2 microglial cells exposed to pro-inflammatory stimuli. Moreover, pretreatment with (Ss)-DS-ONJ increased Heme-oxygenase (HO)-1 as well as interleukin 10 (IL10) expression in LPS-stimulated microglial cells, thereby promoting M2 (anti-inflammatory) response by the induction of Arginase-1. The results strongly suggest that this is the likely molecular mechanism involved in the anti-inflammatory effects of (SS)-DS-ONJ in microglia. (SS)-DS-ONJ further reduced gliosis in retinal explants from type 1 diabetic BB rats, which is consistent with the enhanced M2 response. In conclusion, targeting microglia polarization dynamics in M2 status by compounds with anti-inflammatory activities offers promising therapeutic interventions at early stages of DR.
Keywords: M2 response; diabetic retinopathy; immunomodulation; microglia; sp2-iminosugar glycolipids.
Copyright © 2021 Cano-Cano, Alcalde-Estévez, Gómez-Jaramillo, Iturregui, Sánchez-Fernández, García Fernández, Ortiz Mellet, Campos-Caro, López-Tinoco, Aguilar-Diosdado, Valverde and Arroba.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Caspard H, Jabbour S, Hammar N, Fenici P, Sheehan JJ, Kosiborod M. Recent trends in the prevalence of type 2 diabetes and the association with abdominal obesity lead to growing health disparities in the USA: An analysis of the NHANES surveys from 1999 to 2014. Diabetes Obes Metab (2018) 20(3):667–71. 10.1111/dom.13143 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

