Enhanced Glycolysis Is Required for Antileishmanial Functions of Neutrophils Upon Infection With Leishmania donovani
- PMID: 33815385
- PMCID: PMC8017142
- DOI: 10.3389/fimmu.2021.632512
Enhanced Glycolysis Is Required for Antileishmanial Functions of Neutrophils Upon Infection With Leishmania donovani
Abstract
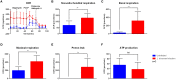
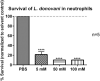
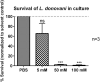
Visceral leishmaniasis (VL) is a fatal parasitic disease if untreated. Treatment options of VL diminish due to emerging drug resistance. Although the principal host cells for the multiplication of Leishmania are macrophages, neutrophils are the first cells infected with the parasites rapidly after parasite inoculation. Leishmania can survive in neutrophils despite the potent antimicrobial effector functions of neutrophils that can eliminate the parasites. Recently, the growing field of immunometabolism provided strong evidence for the therapeutic potential in targeting metabolic processes as a means of controlling immune effector functions. Therefore, the understanding of the immunometabolic profile of neutrophils during Leishmania infection could provide new promising targets for host-directed therapies against VL. To our knowledge, this is the first study addressing the bioenergetics profile of L. donovani-infected primary human neutrophils. Transcriptome analysis of L. donovani-infected neutrophils revealed an early significant upregulation of several glycolytic enzymes. Extracellular flux analysis showed that glycolysis and glycolytic capacity were upregulated in L. donovani-infected neutrophils at 6 h post infection. An increased glucose uptake and accumulation of glycolytic end products were further signs for an elevated glycolytic metabolism in L. donovani-infected neutrophils. At the same time point, oxidative phosphorylation provided NADPH for the oxidative burst but did not contribute to ATP production. Inhibition of glycolysis with 2-DG significantly reduced the survival of L. donovani promastigotes in neutrophils and in culture. However, this reduction was due to a direct antileishmanial effect of 2-DG and not a consequence of enhanced antileishmanial activity of neutrophils. To further address the impact of glucose metabolism during the first days of infection in vivo, we treated C57BL/6 mice with 2-DG prior to infection with L. donovani and assessed the parasite load one day and seven days post infection. Our results show, that seven days post-infection the parasite load of 2-DG treated animals was significantly higher than in mock treated animals. This data indicates that glycolysis serves as major energy source for antimicrobial effector functions against L. donovani. Inhibition of glycolysis abrogates important neutrophil effector functions that are necessary the initial control of Leishmania infection.
Keywords: 2-deoxyglucose; glucose metabolism; glycolysis (glycolytic pathway); host-directed therapy (HDT); leishmania; neutrophils; oxidative phosphorylation (OXPHOS); transcriptome (RNA-seq).
Copyright © 2021 Ohms, Ferreira, Busch, Wohlers, Guerra de Souza, Silvestre and Laskay.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

